3. Key GxPs in Medicine
1. Key GxPs in Medicine
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
Patients expect medicines to be safe, effective, of high quality and accessible.
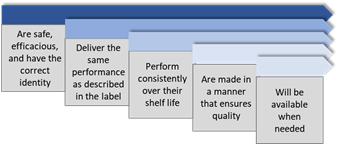
GxP rules and guidelines ensure that all aspects of the medicines development process are conducted according to the best methods for safety, efficacy , and quality.
The most relevant GxPs in the pharmaceutical industry are:
- Good Laboratory Practice (GLP)
- Good Clinical Practice (GCP)
- Good Manufacturing Practice (GMP)
- Good Pharmacovigilance Practice(GVP)
- Good Distribution Practice (GDP)
- Good Documentation Practice (GDocP)
The extent of what GxPs are included in a GxP system often depends on the respective jurisdiction, but GLP, GCP and GMP are always comprised as a minimum. Further, in the legislation some GxPs are subsumed under another GxP, e.g. Good documentation practice (GDP) can be found under Good manufacturing practice (GMP).
Figure 1 below is a high-level overview of Key GxPs along the medicines R&D process. Of note: the Good Lay Summary Practice GLSP is included here among the Key GxPs because it is of particular interest for patients/patient advocates.
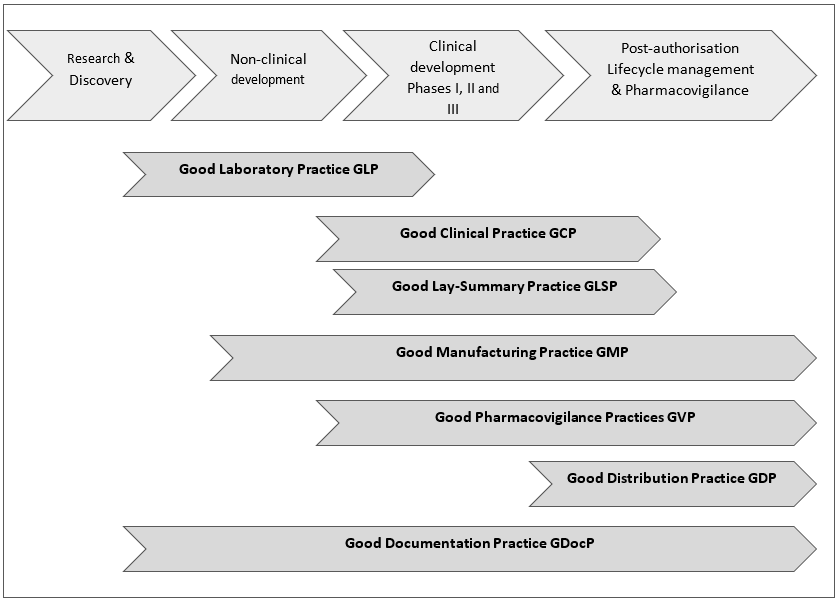
Figure 1: High-level overview of Key GxPs along the medicines R&D process