Early Clinical Development
Topic outline
-
After completing this course, you will be able to:
- Understand the principles of pharmacology, methods of measuring and;
- Describe the types of studies in early clinical development (Phase I and Phase II studies).
EUPATI Open Classroom content is free for all learners.
Enrol & Access the assessment
At the end of each course you can test your knowledge and earn a certificate for a 10-euro administrative fee. If interested, click the button below: -
The early clinical development plan: the objectives, designs including minimisation of bias, conduct and analysis of early exploratory development studies, incl. role of Pos; “Exploratory” and “confirmatory” clinical development versus “Phases I-IV of clinical development”
Clinical Research from ECRAN project.
If you would like to watch this video in any of 18 other languages, please click here: http://vimeo.com/ecranproject/videos/sort:alphabetical/format:thumbnail
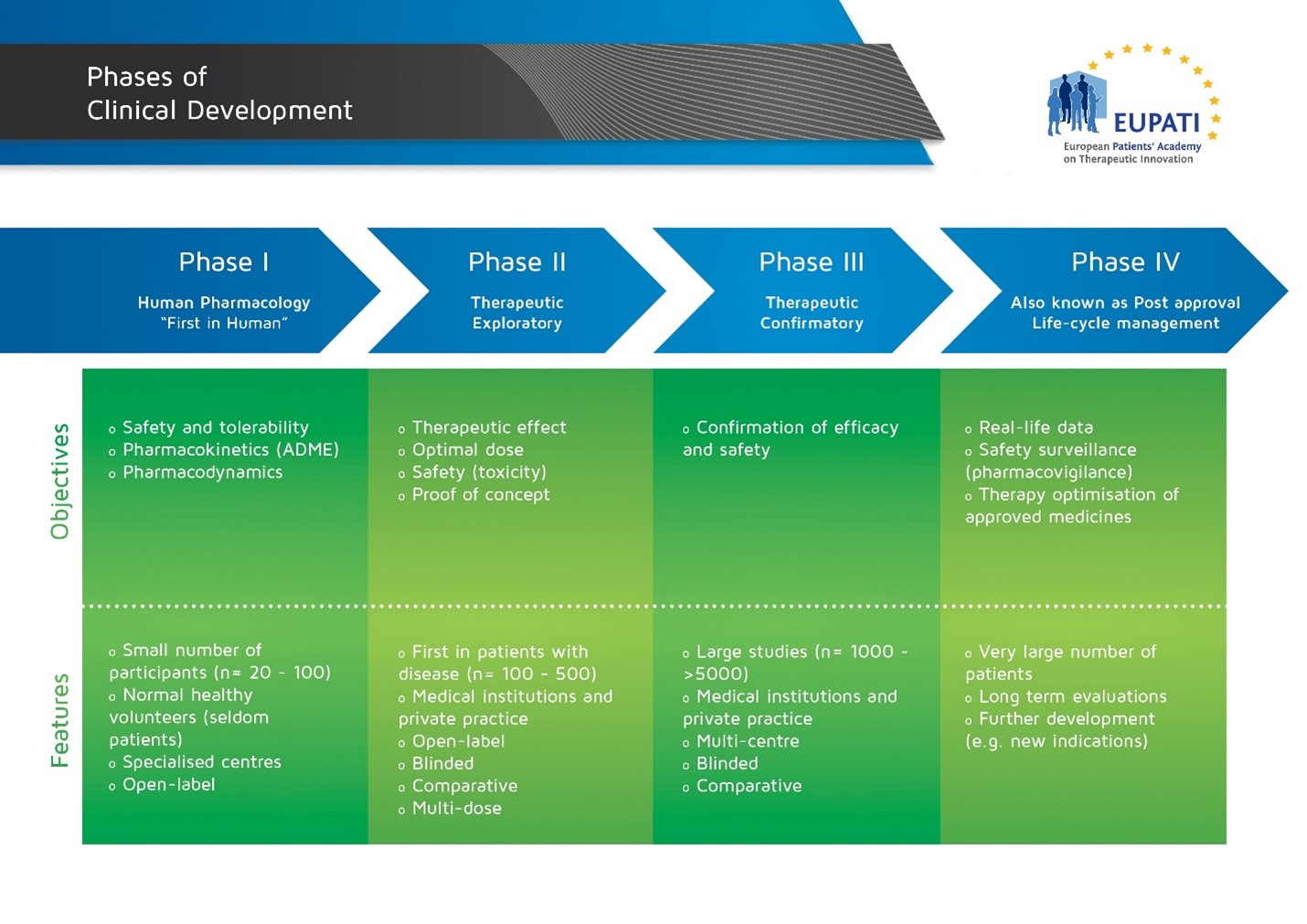
Phases of Clinical Development - image
-
-
The objective of ADME studies is to understand and characterise a medicine’s absorption, distribution, metabolism, and excretion. Although usually associated with Phase I, these studies may be performed throughout the development of a medicinal product. These studies are typically performed using a single dose in healthy males (n=4-6) using the intended route of administration.The information gained from ADME studies is:
- Distribution of parent medicine and metabolites in different body compartments (e.g. after crossing the blood-brain barrier)
- Proportion of parent medicine converted into metabolite(s)
- Primary mechanism(s) of elimination and excretion from the body
-
-
-
-
-
-
-
-
Learners mustReceive a gradeReceive a passing gradeTo receive a certificate for this course, you must be enrolled in the course (click on 'Enrol me in this course' yellow button at the top right on the course page), then complete the assessment and obtain at least 70% of correct answers. You have 10 attempts in total. Good luck!
-
Early Clinical Development
After completing this course, you will be able to:
- Understand the principles of pharmacology, methods of measuring and;
- Describe the types of studies in early clinical development (Phase I and Phase II studies).
Clinical Trials | Pharmacology | Early clinical development | Measurements
Duration: 3 hours
Recommended Courses: 12, 13