4. What is Health Technology Assessment (HTA)?
What is Health Technology Assessment?
Health Technology Assessment (HTA) is a multiscientific and interdisciplinary activity delivering input for priorities and decisions in the health care system in relation to prevention, diagnostics, treatment and rehabilitation. Decisions on the use of technology are made at all levels in the health care system. They often include a unification of complicated medical, patient-related, organisational and economic information in a context where there may also be ethical problems. Decisions must be made on an evidence-based foundation where all relevant circumstances and consequences are systematically evaluated by means of scientific methods.
Health Technology Assessment (HTA) can be considered a policy-informing and decision-making tool and has the potential to contribute to quality of care, equity in access and value for money by providing input to decision-making at different levels in the system and fostering both, the adoption of valuable innovation and the removal of obsolete technologies.
Figure 1 based on: Kristensen FB, Matzen P, Madsen PB. Health technology assessment of the diagnosis of colorectal cancer in a public health service system. Seminars in Colon & Rectal Surgery 2002;13(1):96-102.
The HTA is equally related to the policy and structure of a society as to research and scientific methods.
The political decisions in health care systems based on HTA are rooted in four ethical principles:
- Respect for autonomy - the decisions of the individual regarding his/her life must be respected.
- Non-maleficence - a duty to avoid causing harm intentionally.
- Beneficence - doing good and encouraging good life and balancing the possible benefit of anaction against the possible harmful effects.
- Justice - ensuring a fair distribution of all basic benefits and burdens.
Whereas the first three principles are relevant to the direct patient-doctor relationship (but not only there) the principle of justice concerns the relationship between several persons or groups of people. The question of distribution of resources in a health care system is, for instance, typically a question of justice.
HTA is used by many health systems across the world, and its use is becoming more widespread. However, HTA has been defined in different ways in different systems, sometimes broadly and sometimes more narrowly.
HTA can be described as encompassing four segments: (i) policy analysis; (ii) evidence-based medicine; (iii) health economic evaluation; and (iv) social and humanistic sciences in conjunction with the dimensions, it captures of the use of health technologies. Depending on whether HTA is applied to new or established technologies, it contributes to setting or updating standards for healthcare provision. HTA can therefore be understood as both a quality assurance and an efficiency mechanism.
An international joint task group, consisting of representatives of leading HTA networks, societies and global organisations, co-led by the International Network of Agencies for Health Technology Assessment (INAHTA) and Health Technology Assessment International (HTAi) has developed a new and internationally accepted definition of HTA, agreed by INAHTA, HTAi, HTA Glossary English Editorial Board, ISPOR (International Society for Pharmacoeconomics and Outcomes Research) , RedETSA (Health Technology Assessment Network of the Americas (Red de Evaluación de Tecnologías en Salud de las Américas)), HTAsiaLink (collaborative research network of HTA agencies in the Asia-Pacific region) and EUnetHTA (the European Union Network of Health Technology Assessment organisations), and published in May 2020[1]
The new definition of HTA is shown below, and includes important clarifying information provided in four accompanying notes[2]:
’A multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle. The purpose is to inform decision-making in order to promote an equitable, efficient, and high-quality health system.
Note 1: A health technology is an intervention developed to prevent, diagnose or treat medical conditions; promote health; provide rehabilitation; or organize healthcare delivery. The intervention can be a test, device, medicine, vaccine, procedure, program, or system (definition from the HTA Glossary: HtaGlossary.net | health technology)
Note 2: The process is formal, systematic and transparent, and uses state-of-the-art methods to consider the best available evidence.
Note 3: The dimensions of value for a health technology may be assessed by examining the intended and unintended consequences of using a health technology compared to existing alternatives. These dimensions often include clinical effectiveness, safety, costs and economic implications, ethical, social, cultural and legal issues, organisational and environmental aspects, as well as wider implications for the patient, relatives, caregivers, and the population. The overall value may vary depending on the perspective taken, the stakeholders involved, and the decision context.
Note 4: HTA can be applied at different points in the lifecycle of a health technology, i.e., pre-market, during market approval, post-market, through to the disinvestment of a health technology.’
🔖Here is the link to the definition on the HTA Glossary: HtaGlossary.net | health technology assessment
Generally speaking, HTA aims to inform decisions made in healthcare systems about which health technologies are of most value and should be employed and invested in. This determination of value is complex and must take policy and social context into account.
The current gold standard, at least in Europe, underpinning the procedural and methodological understanding of conducting HTA is the HTA Core Model® (Figure 2), developed in an iterative process over 10 years by the EUnetHTA collaboration and Joint Actions (Kristensen et al., 2017)[3].
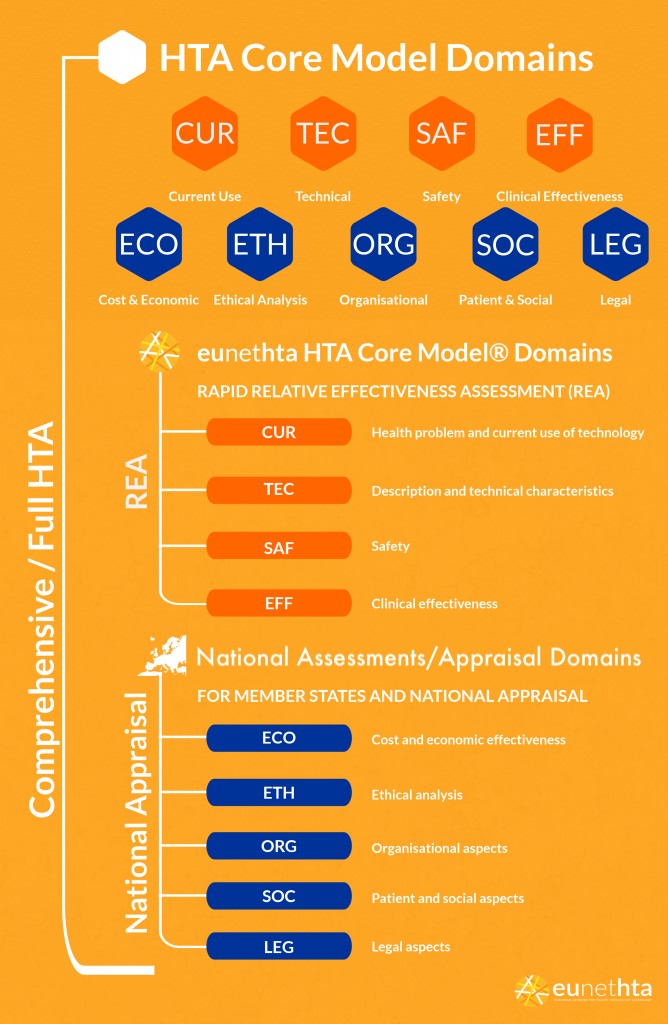
Figure 2 HTA Core Model®
REA, Relative Effectiveness Assessment, is a specific element of HTA that focuses on the clinical benefit of the technology, whereas the Full HTA includes other aspects such as costs, ethical, legal and societal considerations. These aspects reflect national values and structures that differ between countries.
From the EUnetHTA website: “The HTA Core Model® is a methodological framework for production and sharing of HTA information. It consists of three components, each with a specific purpose:
- a standardised set of HTA questions (the ontology), which allow users to define their specific research questions within a hierarchical structure;
- methodological guidance to assist in answering the research questions and
- a common reporting structure for presenting findings in a standardised ‘question-answer pair’ format.”
The HTA Core Model® consists of the following nine domains:
- Health problem and current use of health technology
- Description and technical characteristics
- Safety
- Clinical effectiveness
- Costs and economic evaluation
- Ethical analysis
- Organisational aspects
- Patient and societal aspects
- Legal aspects
Not all domains of the model are necessarily meant to be addressed in all HTA reports; depending on the scope of evaluation foreseen in the decision making process, the technology at hand and feasibility parameters, a narrower or broader perspective can be adopted. Quite often, reports focus on the clinical and economic implications only (Lee, Skött & Hansen, 2009).
References
[1] The new definition of health technology assessment: A milestone in international collaboration | International Journal of Technology Assessment in Health Care | Cambridge Core
[3] Kristensen FB et al. (2017). The HTA Core Model® – 10 Years of Developing an International Framework to Share Multidimensional Value Assessment. Value in Health, 20(2):244–50. Available at: (PDF) The HTA Core Model®—10 Years of Developing an International Framework to Share Multidimensional Value Assessment (researchgate.net)