5. EU Regulatory procedures for a marketing authorisation (MA)
1. EU Regulatory procedures for a marketing authorisation (MA)
1.4. Non-centralised procedures:The Mutual recognition procedure and the decentralised procedure
Common characteristics
- The mutual recognition procedure or the decentralised procedure must be used for applications for marketing authorisation for medicines intended for marketing in more than one Member State
- Both procedures are based on the recognition by national competent authorities of an assessment performed by the authorities of one MS based on an identical dossier in these Member States.
- The procedures aim at facilitating access to a single market comprising the involved CMSs[1] by relying upon mutual recognition.
- It is not necessary to apply for a MA in all 27 MSs – a minimum of two is required.
- The application dossier is assessed by one member state (reference member state, RMS), the review is shared with other involved member states (concerned member state, CMS).
- The company has the choice of RMSs and CMS(s).
- The company has the choice of trade names.
- At the end of the procedure a national MA is issued by each member state involved, which includes a harmonised SmPC, labelling and Package leaflet (PL).
- Consideration should be given to the need for ‘user consultation’.
- In principle, CMSs should rely on the assessment of the RMS. Concerns may nevertheless be raised on grounds of a potential serious risks to public health. The issue, if not resolved between CMS, RMS and applicant, will be referred to the coordination group (CMD(h)). MSs shall reach a consensus within 60 days. In case this fails, the procedure is submitted to the EMA scientific committee (CHMP) for arbitration (Art. 29, Directive 2001/83/EC).
- Any application to vary a marketing authorization which has been granted under the MRP or DCP needs to be submitted to all the Member States which have previously authorised the medicine concerned (Art. 35 of Directive 2001/83/EC)
- The procedures are overseen by the Heads of Medicines Agencies (HMA) via the Coordination Group for Mutual Recognition and Decentralised Procedures - Human (CMD(h)).
1. Mutual recognition procedure - MRP
- The mutual recognition procedure is based on the principle of the mutual recognition by CMSs of a national MA where the medicine in question has received a MA in any Member State at the time of application.
- An identical application for mutual recognition is to be submitted to all concerned MSs and the applicant requests one Member State to act as RMS.
- The RMS either is asked to prepare an assessment report on the medicine or, if necessary, to update any existing assessment report.
- As soon as the AR is completed, copies of this report are sent to all CMSs, together with the approved SmPC, labelling and package leaflet.
- The concerned Member States shall recognise the marketing authorisation granted by the RMS.
- National marketing authorisations shall be granted within 30 days after acknowledgement of the agreement.
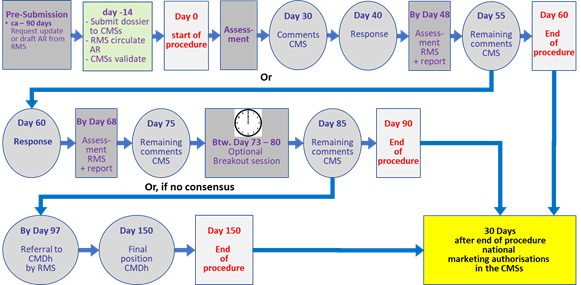
The following table (Table 2) and flowchart (Fig.5) give an overview and timeline of the MRP.
Table 2: Brief overview of temporal and operational sequence of the mutual recognition procedure (MRP) for marketing authorisations in more than one member state (MS).
|
Approx. 90 days before submission to CMS(s) |
Applicant requests RMS to update Assessment Report (AR) and allocate procedure number. |
|
14 days before start of procedure |
Applicant submits dossier to CMS(s). RMS circulates AR including SmPC, PL and labelling to CMSs. Validation of the application by CMSs. |
|
Day 0 |
RMS starts procedure |
|
Day 30 |
CMSs send their comments to the RMS, CMSs and applicant |
|
Day 40 |
Applicant sends the response document to CMSs and RMS |
|
Until Day 48 |
RMS evaluates and circulates a report on the applicant’s response document to CMSs. |
|
Day 55 |
CMSs send their remaining comments to RMS and applicant. |
|
Day 55-59 |
The applicant and RMS are in close contact to clarify if the procedure can be closed at day 60 or if the applicant should submit a further response at day 60. |
|
Day 60 |
If CMS have no remaining comments at Day 55, the RMS closes the procedure. If a CMS has remaining comments at Day 55, the applicant sends the response document to CMSs and RMS |
|
Day 60 - 90 |
Only used if a CMS has remaining comments |
|
Until day 68 |
RMS evaluates and circulates a report on the applicant’s response document to CMSs |
|
Day 75 |
CMSs send their remaining comments to RMS, CMSs and applicant. |
|
Until Day 80 |
Optional break-out session (BOS) can be organised around Day 75 (but may take place between days 73-80) |
|
Day 85 |
CMSs send any remaining comments to RMS, CMS and applicant |
|
Day 90 |
CMSs notify RMS and applicant of final position (in case of negative position also the CMDh secretariat of EMA). If consensus is reached, the RMS closes the procedure.
If consensus is not reached, the points for disagreement submitted by CMSs are referred to CMDh by the RMS within 7 days after day 90 |
|
Day 150 |
Final position adopted by the CMDh: If consensus is reached at the level of CMDh, the RMS closes the procedure. If consensus is not reached at the level of CMDh, the RMS refers immediately the matter to EMA for CHMP arbitration |
|
7 days after close of procedure |
Applicant sends high quality national translations of SmPC, PL and labelling to CMSs. |
|
30 days after close of procedure |
Granting of national marketing authorisations in the CMSs subject to submission of acceptable translations |
- The DCP is available for new products in cases where the medicine has no marketing authorisation at the time of application.
- It is a single, streamlined procedure with the possibility for shortened approval times in straightforward cases.
- It is possible to end the procedure at any time point during the procedure if consensus is reached.
- Submission of an application in all the MSs involved at the same time.
- One MS acts as the RMS and takes the lead (this can be suggested by the sponsor)
- The RMS prepares a draft assessment report, including SmPC, labelling and PL and sends them to the CMSs and to the applicant.
- The CMSs shall approve the assessment report, the SmPC and the labelling and PL.
- National MAs will be granted within 30 days after acknowledgement of the agreement in the RMS and each CMS with a harmonised SmPC, labelling and PL
The following table (Table 3) and flowchart (Fig. 6) give an overview and timeline of the DCP.
Table 3: Brief overview of temporal and operational sequence of the decentralised procedure (DCP) for marketing authorisations in more than one member state (MS)
|
Pre-procedural Step |
|
|
14 days before submission |
Applicant discussions with RMS. RMS allocates procedure number |
|
14 days before start of procedure |
Submission of the dossier to the RMS and CMSs. Validation of the application. |
|
Assessment step I |
|
|
Day 0 |
RMS starts the procedure. CMSs are informed |
|
Day 70 |
RMS forwards the Preliminary Assessment Report (PrAR) (including comments on SmPC, PL and labelling) to the CMSs and the applicant. |
|
Until Day 100 |
CMSs send their comments, if there are any, to the RMS, CMSs and applicant |
|
Until Day 105 |
Consultation between RMS and CMSs and applicant. If consensus not reached RMS stops the clock to allow applicant to supplement the dossier and respond to the questions. |
|
Clock-stop period |
Applicant sends final response document to the RMS and CMSs within a period of 3 months, which can be extended by a further 3 months. |
|
Day 106 |
RMS restarts the procedure after receipt of a final response or expiry of the agreed clock-stop period without a response. CMSs are informed |
|
Assessment step II |
|
|
Day 120 (Day 0) |
RMS sends the draft AR, including SmPC, labelling and PL to CMSs and the applicant. |
|
Day 145 (Day 25) |
CMSs send comments, if there are any, to RMS, CMSs and the applicant. |
|
Day 150 (Day 30) |
RMS may close procedure if consensus reached. CMSs then have 30 days to grant the MA |
|
Day 160 |
Applicant sends the response document to CMSs and RMS. |
|
Until 180 (Day 60) |
If consensus is not reached by day 150, RMS to communicate outstanding issues with applicant, receive any additional clarification, prepare a short report and forward it to the CMSs and the applicant. |
|
Day 195 (at the latest) |
A Break-Out Session (BOS) may be held at the EMA (or via TC) with the involved MSs to reach consensus on the major outstanding issues. |
|
Between Day 195 and Day 210 |
RMS consults with the CMSs and the applicant to discuss the remaining comments raised. |
|
Day 210 (Day 90) |
If consensus is reached: - In case of positive position from RMS, Closure of the procedure including End of Procedure letter, final Day 210 overview AR, SmPC, labelling and PL, active substance and finished product specifications and proceed to national 30 days step for granting the MA. - In case of negative position from the RMS, closure of the procedure negatively. The End of Procedure letter and final Day 210 overview AR is circulated. If consensus is not reached: In case of negative position from CMS, CMS notifies the RMS, the other CMSs, applicant and the secretariat of the CMDh. Referral to the CMDh. |
|
At the latest, within 7 days after Day 210 |
If consensus on a positive RMS AR was not reached at day 210, the points of disagreement submitted by CMS(s) will be referred by the RMS to the CMDh for resolution. |
|
Day 270 (at the latest) |
Final position adopted by CMDh with referral to CHMP for arbitration in case of unsolved disagreement. |
* The Communication and Tracking System (CTS) is the system used by the National Competent Authorities (NCAs) involved in the licensing of human and veterinary medicinal products via the MRP and DCP. CTS supports the co-ordination and tracking of marketing authorisation, post-licensing and work sharing procedures as monitored by the Coordination Groups for MRP and DCP. The system serves as data provider for other applications.
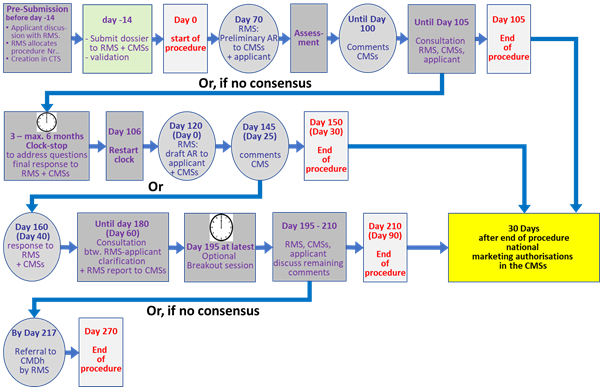
Figure 7: Flowchart of the decentralised procedure (DCP)
Legal basis for MRP and DCP: Artl. 8 (1), (3); 17; 10, 10a,b,c; 11; 18; 28 to 39, Directive 2001/83 (EC)
[1] A single market is referring to a multitude of entities still being considered as ‘one’. For example, the EU market is a single market.