What is pharmacovigilance (PhV)?
Completion requirements
View
2. How did pharmacovigilance develop?
- One of the earlier reported events was when a young girl, Hannah, died after chloroform anaesthetic before removal of an infected toenail. While the cause of death was not determined at that time, it did begin to raise awareness of adverse events related to anaesthesia. It’s interesting to think about how many centuries went by without this type of detection or oversight.
- US and FDA: 1906, the US Federal Food and Drug Act provided the basic elements for consumer protection. 1911, regulation against false therapeutic drug indications. 1937, there were more than 100 deaths associated with diethylene glycol as a solvent used in a sulfanilamide elixir. Public outcry led to the creation of the Food, Drug and Cosmetic Act. Its aim was to renovate the public health system and introduce protections regarding the safety of drugs before their market approval.
- WHO: 1968, the World Health Organisation (WHO) initiated its Programme for International Drug Monitoring in response to the thalidomide disaster detected in 1961 .
'From these beginnings emerged the practice and science of
pharmacovigilance. Systems were developed in Member States for the collection
of individual case histories of ADRs (Adverse Drug Reactions) and evaluation of
them. The collection of international ADR reports in a central database, would
serve the important function of contributing to the work of national drug
regulatory authorities, improve the safety profile of medicines, and help avoid
further disasters.’
- EU and EMA: 1995, the European Medicines Agency (EMA) was created. 2004 Introduction of Risk Management Plans (RMP) and review of EU PhV system. 2010/12 New PhV legislation operational (amending Regulation 726/2004 and Directive 2001/83 and Commission Implementing Regulation (IR) 520/2012) and Pharmacovigilance Risk Assessment Committee (PRAC) established, 2017 new enhanced EudraVigilance (EV) system.
All these events (and more) and legislative changes paved the way for increased awareness regarding the conduct of Pharmacovigilance activities to safeguard patient safety throughout the lifecycle of medicines.
While there are many significant historical events that contributed to the genesis of PhV, the exemplary milestones shown in the following graph are just a few of the significant ones:
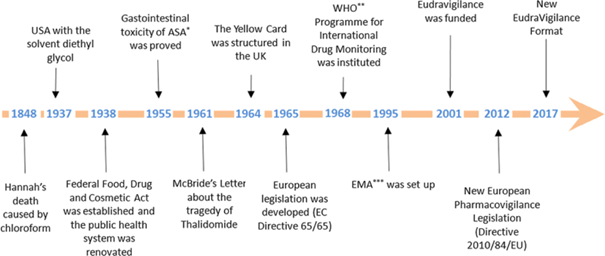
Figure 1: Timeline of the historical evolution of
Pharmacovigilance.
*ASA:
acetylsalicylic acid; **WHO: World Health Organisation; ***EMA: European
Medicines Agency