2. About Good 'x' Practice (GxP)
1. About Good 'x' Practice (GxP)
1.2. Compliance and inspections
All organisations involved in the development, marketing, manufacture and distribution of medicines are responsible for ensuring that they comply with all relevant standards set out in European Union (EU) legislation and guidelines on pharmaceuticals. The GxP requirements outlined by the respective authorities, ask companies to formally define controlled processes tools and quality systems, irrespective of the operational sector and the specific GxP standards that apply, to demonstrate for example:
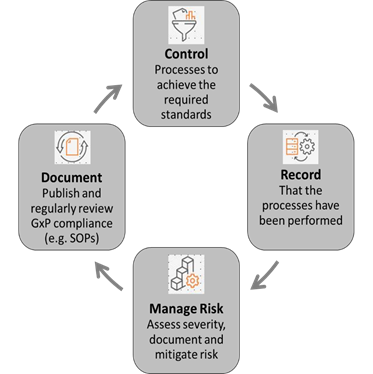
- How new health technologies are researched and developed
- How products are consistently manufactured
- How products are tested
- How laboratory and manufacturing equipment is calibrated and maintained
- How possible risks are identified and mitigated
- How processes are traced from start to finish through controlled documentation
- How different kinds of products are stored and distributed
- How those involved in the processes are trained
- How records of all these processes are captured and maintained to monitor continued effectiveness of all respective processes and systems
GxP compliance is monitored and enforced by agencies and government bodies through regular inspections and unannounced auditing, and certification requirements where applicable (Medical Devices). For companies this includes also to verify and vouch for the GxP compliance of their partners throughout the supply chain.
In many cases the scope of the regulator’s remit continues to widen taking in new sectors and product categories. This reflects the extent of innovation in these areas, as well as cross-sector dependencies in the techniques they use.
The European Medicines Agency (EMA) is responsible for harmonising the standards at EU level. The standards are constantly updated, developed and agreed, under the coordination of the EMA, by representatives of the GMP Inspectorates of each Member State. Guidelines on inspections of pharmacovigilance of medicinal products are part of the Good Vigilance Practice guidelines adopted by the EMA. Guidelines on GCP inspections are part of EudraLex, Volume 10.
The EMA coordinates inspections for medicines authorised under the centralised procedure or in the context of a referral, on request from the Committee for Medicinal Products for Human Use (CHMP).
EMA does not conduct inspections itself but inspections are carried out by the national competent authorities in the EU Member States. Inspections are performed regularly on sites within and outside the EU involved in developing, manufacturing and distributing medicines intended for the EU market, to verify their compliance with the relevant standards. An inspection may either be 'for cause', when it is triggered by a finding of possible non-compliance with relevant standards, or routine, when inspections are carried out as part of a surveillance programme.
Inspections are conducted both for authorised medicines and for medicines under evaluation in the EU. These inspections ensure that the rights, safety and wellbeing of clinical-trial participants are protected, and the reliability and integrity of data that support the authorisation of medicines and their quality, safety and effectiveness once on the market is duly controlled.
Legal basis:
Regulation
(EC) No 726/2004 Articles 8, 19, 57.1(i)
Council Directive 2001/83/EC Article 111
Member States are obliged to take account of the Compilation* by virtue of
Article 3(1) of Directive (EU) 2017/1572 and Article 17.1 of Regulation (EU)
2017/1569.
*Compilation of Union Procedures on Inspections and Exchange of Information
Table 1: Brief overview of inspections adapted from EMA https://www.ema.europa.eu/en/human-regulatory/overview/compliance-overview#inspections:-verifying-compliance-section
|
Inspections |
GCP |
GMP |
GLP |
GVP |
|
Purpose of inspections |
Confirms that clinical trials supporting a marketing authorisation application are conducted in line with GCP standards and all relevant EU legal and regulatory requirements |
Confirms that any medicine intended for the EU market is manufactured in compliance with GMP standards and all relevant EU legal and regulatory requirements |
Confirms that non-clinical studies supporting a marketing authorisation application are conducted compliant with GLP standards and all relevant EU legal and regulatory requirements |
Confirms that the marketing authorisation holder has personnel, systems and facilities to meet their pharmacovigilance obligations |
|
Sites in scope |
Any site where clinical trials that are part of an application are carried out, no matter where in the world it is located. |
· Any site manufacturing a medicine intended for the EU market, no matter where it is located · Sites in the EU are routinely inspected by the national authority · Typically performed at manufacturing sites located in third countries, for which a mutual recognition agreement (MRA) with the EU does not apply |
Any site where pivotal non-clinical studies are carried out, no matter where in the world it is located |
Any party carrying out pharmacovigilance activities in whole or in part, on behalf of or in conjunction with, the marketing authorisation holder, no matter where in the world it is located |
|
When they occur (on CHMP request) |
At any stage in the evaluation of a medicine. |
At any time during a medicine's evaluation or after authorisation in the EU. |
At any stage in the evaluation of a marketing authorisation application. |
At any stage during the evaluation of a marketing authorisation application or after a medicine is authorised. |
|
Impact of adverse outcomes |
If a GCP inspection reports critical or major findings on the conduct of clinical trials: · the CHMP evaluates the impact of the findings on the medicine’s benefit-risk balance and on the rights, safety and wellbeing of clinical trial participants · the CHMP may request analyses of clinical trial data excluding the affected patients or sites |
If a GMP inspection leads to adverse findings, the company must implement a corrective action plan agreed with the inspectors. |
If inspectors report critical or major findings on the conduct of non-clinical studies, the CHMP evaluates the impact on the medicine’s benefit-risk balance. |
|
|
Publicly available information |
Each medicine's assessment report includes information on the: · types and locations of sites inspected; · any impact of inspection findings on the benefit-risk assessment of the medicine. |
The EudraGMDP database holds the data collected during GMP inspections by EU authorities. Each medicine's assessment report includes information on the: · types and locations of sites inspected; · any impact of inspection findings on the benefit-risk assessment of the medicine. |
Each medicine's assessment report includes information on the: · types and locations of sites inspected; · any impact of inspection findings on the benefit-risk assessment of the medicine. |
|
|
International cooperation |
If the clinical trials took place outside the EU, and if EMA has a confidentiality arrangement with the responsible non-EU regulator, the two jurisdictions may: · exchange information on inspection findings and their interpretation; · conduct collaborative inspections. |
If a medicine is manufactured outside the EU, and if the EU has a mutual recognition agreement (MRA) with the country in question, the two jurisdictions can: · rely on each other's GMP inspection system; · share information on GMP inspections and quality defects;waive batch testing of products on import into their territories; EMA also takes part in international initiatives to: · exchange information on inspection planning and outcomes; · conduct joint inspections for manufacturing sites of common interest. |
Fpr non-clinical studies outside the EU, and if EMA has a confidentiality arrangement with the responsible non-EU regulator, the two jurisdictions may: · exchange information on inspection findings and interpretation; · conduct collaborative inspections. The EU is a signatory to the OECD mutual acceptance of data (MAD) agreement, which allows participating authorities to rely on each other's monitoring programmes for GLP compliance. |
EMA can exchange information on pharmacovigilance inspection findings with non-EU regulators with which EMA has signed a confidentiality arrangement. |