3. Processes and methods
1. Processes and methods
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
There is great variation in the scope, selection of methods and level of detail in the practice of HTA. In a condensed form the HTA is part of a process which can be characterised by three phases (Figure 1):
- Assessment: Is a phase of collation and critical review of scientific evidence and consideration of clinical and all other factors (economic, legal, ethical, etc.) by the HTA body to make a recommendation.
- Appraisal: Is a phase in the process following the assessment when recommendations on the use of health technology are given. This phase also includes value judgement.
- Decision-making and implementation: based on the appraisal of the recommendation a decision is made, usually by a different body (e.g. a committee appointed by the ministry of health). The technology is implemented in the health system and its impact is monitored.
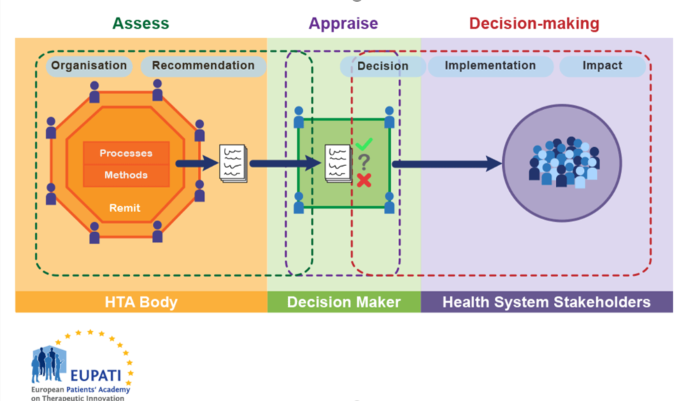
Figure 1. HTA involvement in the Health System
HTA bodies must choose what information is important for decision makers to have about a technology and how they will gather that information. How an HTA body defines its processes and methods influences the assessments, and they in turn are influenced by the organisation and remit of the HTA body itself.
Most activities involve some form of the following basic steps:
- Identify assessment topics.
- Specify the assessment problems or questions.
- Retrieve available relevant evidence.
- Generate or collect new evidence (as appropriate).
- Appraise/interpret quality of the evidence.
- Integrate/synthesise evidence.
- Formulate findings and recommendations.
- Disseminate findings and recommendations.
- Monitor impact.
Not all HTA bodies conduct all of these steps, and they are not necessarily conducted in this order.
For instance, while understanding the clinical effectiveness of a health technology is generally considered important for decision-makers, some health technologies may have ethical issues associated with their use while others do not. An HTA body must choose if it will apply one standard process to all health technologies, or if it will allow for specific processes for the assessment of each technology individually, based on the relevant information required. In this example, should ethical information be gathered for all technologies under evaluation, or should there be a separate process that provides that information where necessary?
An HTA body may employ a variety of methods. Two of the main types are primary data collection methods and secondary or integrative methods. Primary data methods involve collection of original data, such as from clinical trials and observational studies. Integrative methods, or secondary or synthesis methods, involve combining data or information from existing sources, including from primary data studies. Economic analysis methods can involve one or both. Many HTA bodies rely largely on integrative methods of reviewing and synthesising data (using systematic reviews and meta-analyses) based on existing relevant primary data studies (reported in journal articles or from epidemiological or administrative data sets) to formulate findings. Their evaluations are based on distinguishing between stronger and weaker evidence drawn from available primary data.
Some assessment efforts involve multiple cycles of retrieving/collecting, interpreting, and integrating evidence before completing an assessment.
It is not always possible to conduct an assessment on the basis of the most rigorous types of studies. Real world data can only be collected after a medicine has been put on the market. Indeed, policies often must be made in the absence, or before completion, of definitive studies. Given their varying assessment orientations, resource constraints and other factors, HTA bodies tend to rely on a combination of different methods.
To assess a technology, the HTA body usually requires evidence that addresses multiple issues, including:
- The burden of the illness.
- Projected epidemiological trends of a disease.
- The relative effectiveness of technologies.
- The cost-effectiveness of technologies (note that not all HTA bodies consider cost-effectiveness. Some focus more on added clinical benefit (added value) and budget impact).
- How patients value the outcomes of therapy.
In some cases, an HTA body may answer these questions directly or by commissioning new research. In other cases, they may ask the medicine’s marketing authorisation holder (MAH) to provide the relevant information.