3. Processes and methods
1. Processes and methods
1.2. Appraisal
HTA processes generally result in a recommendation to list or not to list the new technology for reimbursement in an insurance-based system (the list includes products with reimbursement from the public health insurance), or recommend it for use in a taxation-based national health service. This may be a listing/recommendation for use of the product under restricted conditions, for example, for a smaller population of patients with more severe illness.
Consider the following diagram:
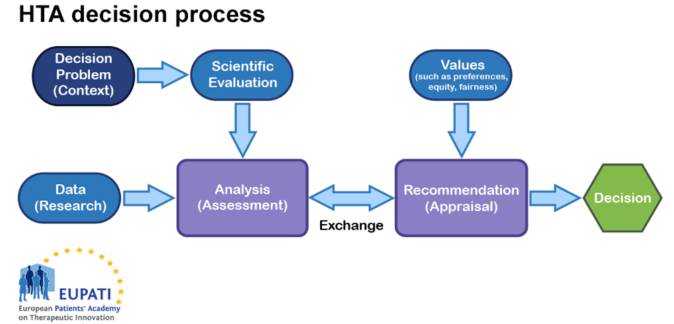
Figure 1: Diagram of HTA process
Determining whether an intervention will reduce heart attack rates, cause significant side effects, or increase healthcare costs requires judgements about the robustness of evidence. There are always uncertainties in the evidence. Clearly, it is in the best interests of any HTA body to use sound scientific judgment and consistent, transparent approaches that lead to defensible decisions. Given the multidisciplinary nature of HTA, the best approaches from epidemiology, sociology, economics, ethics, law, etc. to support the various analyses are required.
Making a decision, however, requires recognition of what society and patients value. Is it a good thing to reduce heart attack rates? At what cost for the health system or, even broader, to society? Although an individual, or analyst, could create a recommendation, it is generally acknowledged that this would be a weak approach. It is unlikely that an individual is capable of covering all of the perspectives and values of those that ultimately will be affected by the decision.
Good approaches to appraisals will consider multiple perspectives and for this reason, a committee is convened that uses an explicit and transparent process to arrive at a recommendation. This process is often called deliberative appraisal.
Most HTA bodies place great emphasis on the magnitude of (and strength of evidence for) gains in patient-relevant health outcomes seen in well-designed clinical trials with appropriate comparators.
The next most important aspect often comprises economic considerations. Almost all HTA bodies consider budget impact (the total amount that the use of the new medicine will add to the health system budget over a defined period). This should be a net budget figure i.e., one that deducts the savings that might occur elsewhere in the health system as a result of benefits associated with the new medicine (e.g. fewer hospital admissions due to an improved side effect profile) from the expected cost.
For the vast majority of technologies, incremental health benefits come with costs to individuals or the healthcare system, and potential implications for resource allocation by individuals and by societies. It should be considered that health improvements may not yield reductions in expenditures within the healthcare system, but these costs might be at least partially offset by savings in other areas of a society's budget. Very difficult decisions need to be made about how to spend a finite health budget, bearing in mind long-term implications for societal health benefits.
The neutrality of the committee structure must be ensured: – in other words, members of the committee must formally declare any possible conflicts of interest or decline their participation.
Some HTA bodies have adopted an ethical framework that allows for their recommendations to be reviewed by a broader set of stakeholders. This allows for an appeal by the pharmaceutical company, clinicians or patients, who may be unjustly impacted by a flawed, biased or imprecise recommendation.
Rarely, HTA bodies seek citizens’ views about challenging aspects of decision-making when deciding priorities in healthcare. For example, in the UK the National Institute for Health and Clinical Excellence (NICE) has a Citizens’ Council that uses a citizens’ jury approach to provide social value judgements that can inform the NICE appraisal committees. The list below details some of the issues that the Citizens’ Council has advised on.

Topics considered by NICE’s Citizens council.
In some cases, the HTA process will be linked to price negotiations. Negotiating price is one mechanism for governments to provide access to new therapies (that is, finding a way not to say ‘no’). Other variables include restrictions on who may be able to receive the treatment under reimbursement schemes.