Principles of New Trial Designs and their Practical Implications
Completion requirements
View
3. Possible Approaches in Adaptive Design
3.2. Example 2: Multi-arm, Multi-stage Design (MAMS)
The multi-arm, multi stage (MAMS) trial is a new paradigm for conducting randomised controlled trials which makes use of an interesting adaptive design.
MAMS trials allow the simultaneous assessment of a number of research treatments against a single control arm. MAMS trials provide earlier answers and are potentially more cost-effective than a series of traditionally designed trials.
In this example, we see a design that uses multiple arms and stages at the same time.
The MAMS design requires a definitive primary and intermediate primary outcome measure. The definitive outcome measure is the one upon which the final conclusions should be based; the intermediate outcome measure provides a means of screening for emerging
evidence of evidence.
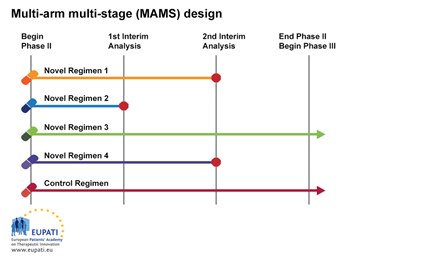
Multi-arm multi-stage design
The multi-arm multi-stage design (MAMS) allows multiple treatments to be tested simultaneously against a single control.
At the first interim analysis in the example above, Novel Regimen 2 is considered to lack sufficient benefit compared with the control and is not taken forward to stage 2. At the second interim analysis, recruitment to Novel Regimens 1 and 4 is stopped, and only the control regimen and Novel Regimen 3 are continued to the end of trial and advanced into Phase III studies.
Advantages of the MAMS design
- Fewer participants
In this design, several trials are performed at once, which helps reduce the number of participants randomised to the control arm. - Less overall time required for medicine discovery
The intermediate steps of the MAMS design replace the separate Phase II step. The decision on whether the medicine is sufficiently active is incorporated as a pilot phase into this trial. - Fewer applications and approvals required
Regulatory work is done for one trial instead of for multiple trials. - Flexible
Uninteresting arms can be dropped and new arms can be added. - Reduced cost
This trial design requires fewer participants, fewer regulatory applications, and less overall time, all of which help to save on development costs.
Disadvantages in MAMS design
- Operating characteristics
Because of the complexity of this approach, it may be difficult to manage and requires a lot of simulations during the design process. - Required number of participants
This depends on the operating characteristics, but if treatment arms are added during the course of the trial, it may be difficult to predict budget and regulatory issues. - Trial duration
If treatment arms are added, it becomes difficult to predict when the trial will naturally end. - Continued accrual (recruitment) to control arm
In order to avoid a time bias when new treatment arms are added, recruitment to the control arm must continue throughout the course of the trial. Consideration must also be given to what happens if a new standard of care becomes available during the course of the trial – is the control still relevant? - Comparison between experimental arms
The MAMS design only allows for comparisons between individual treatment arms and the control arm; it does not allow for comparison between individual treatment arms themselves.