Principles of New Trial Designs and their Practical Implications
Completion requirements
View
3. Possible Approaches in Adaptive Design
3.1. Example 1: Group Sequential Design
A group sequential design is a typical example of a Phase III trial with rules for early stopping for futility or efficacy. In the example trial depicted in the diagram below, patients were randomised between the first line treatment with either one medicine alone or two medicines in combination.
There were two interim stages where it was possible to stop the trial early and performing analysis before all the trial results are gathered. The trial could have been stopped:
- At Interim 1, for futility based on progression-free survival (PFS) – whether the patient stays free of any progression of a specific cancer or not.
- At Interim 2, for futility or efficacy based on overall survival.
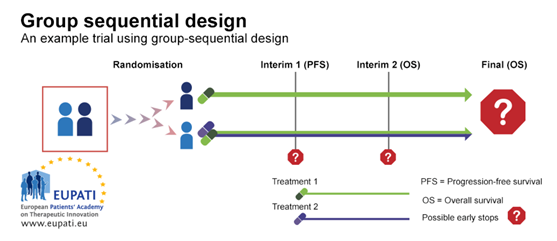
Group
sequential design
Group sequential design allows for early stops on the basis of progression-free
survival or overall survival. In this example, participants were randomised
onto one of two arms, and received either Treatment 1, or a combination of
Treatment 1 and Treatment 2.