Types of Trial Design
Completion requirements
View
3. Randomised Controlled Clinical Trial Designs
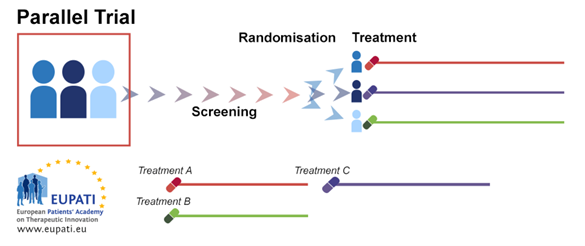
3.3. Parallel Group Trial Design
In parallel group randomisation, participants are randomised to one or two or more arms (groups), there should be an equal number in each arm. Participants receive the same treatment throughout the trial. The results are then compared. Unexplained variability
is attributed to differences between participants. Covariates may explain some participant variables (age, weight, disease severity). The parallel group is the most common design.
Advantages
- Can be applied to almost any disease.
- Any number of groups can be run simultaneously.
- Groups can be in separate locations.
Disadvantages
- High variance brought on by loose connection to control.
- In multiple treatment groups statistics may become challenging.