The Predictive Value of Non-Clinical Testing
Completion requirements
View
5. Toxicology Studies
Toxicology studies examine the safety profile of the development candidate. They also provide important information about the absorption, distribution, metabolism, and excretion (ADME) of the active substance in the body. A medicinal product must be assessed in many different kinds of non-clinical toxicology studies before it can beadministered to the first human volunteer. And before the medicine receives a marketing authorisation more toxicology studies are required.
The following gives an overview of the different types of toxicology studies that may be needed in a non-clinical programme.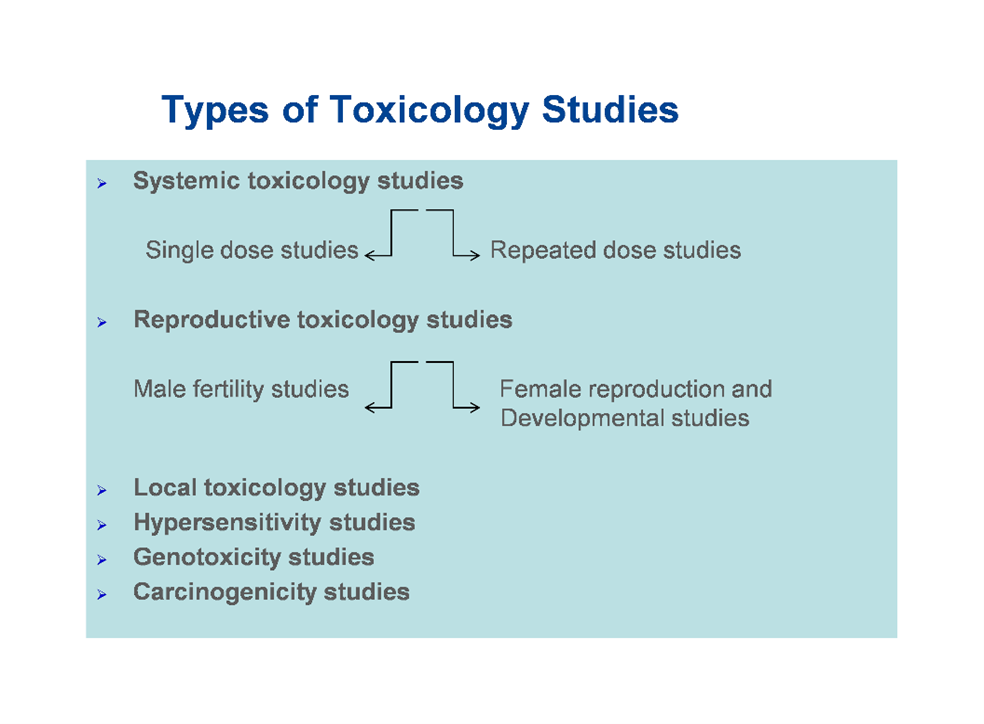 Figure 3: Types of toxicology studies in a non-clinical testing programme.
Figure 3: Types of toxicology studies in a non-clinical testing programme.