The Predictive Value of Non-Clinical Testing
2. From laboratory and animal studies to patients
2.1. Ethical Considerations
The Declaration of Helsinki has defined when it is acceptable to use animal models to assess the risk in humans and to mimic human diseases. It also defines when a development candidate can be given to healthy volunteers from an ethical and scientific point of view. The declaration states that biomedical research should be based on laboratory and animal experiments that are adequately done. Also, the research should be based on deep knowledge of the scientific literature. Researchers must respect the welfare of animals used in the research.
Figures 1 and 2 show how important non-clinical studies are related to clinical trial data during the medicines development process. As shown in Figure 1, researchers rely most on data from the general toxicology studies until late in clinical development. These studies monitor target organs and establish adequate safety margins of exposure. When the medicine is available for marketing, most of the non-clinical data will then be replaced by data from clinical trials.
However, for some aspects of adverse effects, such as a medicines influence on cancer development or in reproduction, it is not ethical to perform the studies directly in humans. In such situations, the clinical use of new medicines might be directed by the non-clinical data for longer periods after marketing approval. This is shown in Figure 2.
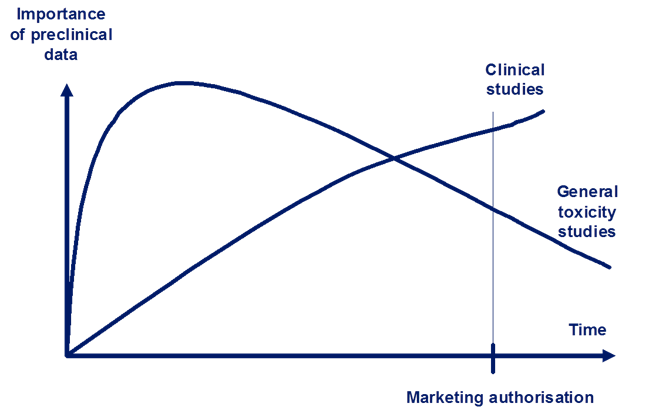
Figure 1: The relative importance and reliance of non-clinical data for human safety. Data from general toxicity studies are relied upon more than clinical data until late in the development process (intersection of the two lines).
Adapted from Non-clinical Assessment Requirements. Maria Nieto-Gutierrez; Safety and Efficacy of Medicines/Human Medicines Development and Evaluation.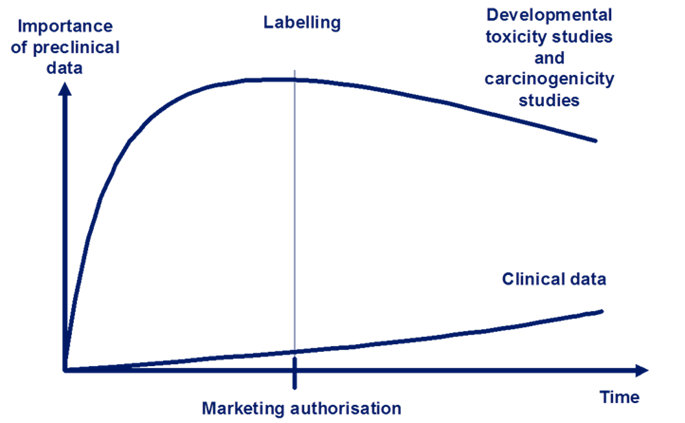
Figure 2: The relative importance and reliance on non-clinical data for human safety. Data from developmental toxicity studies and carcinogenicity studies continue to be relied upon more than clinical data throughout development and after marketing authorisation. Adapted from Non-clinical Assessment Requirements, Maria Nieto-Gutierrez; Safety and Efficacy of Medicines/Human Medicines Development and Evaluation.