2. Patient involvement roadmap for Medical Device development
Patient involvement roadmap for Medical Device development
The development of Medical Devices should involve patients and the public throughout each stage. This is essential in order to ensure that Medical Devices:
- Address a need which patients and the public have identified in order to be of utility and benefit for those who will be using that device in the future.
- Fit for purpose. A device which is developed without reference to user consultation cannot be fit for purpose in terms of ease of use, acceptability, affordability and compatibility with other technologies.
Currently, the development phase of Medical Devices does not promote active patient participation (in contrast to medicinal products), and thus the ability of patients to participate in the development of Medical Devices is quite limited. One of the main objectives is the collection of clinical data that demonstrates the proposed scientific premise. Patients can also be engaged in very practical activities: time (hour in the day) and frequency of appointments for pre-treatment and post-treatment follow-up at the hospital (what is the most desirable and doable way to ensure maximum compliance), modalities of biological sample collection (e.g., blood), and biological examinations (e.g., imaging) when part of the protocols. It is important to emphasize that their participation must be present in all phases of the development of the devices.
The following examples are included in the FDA guidance released on 26 January 2022 [1]:
- Working with patient advisors to improve the informed consent document to ensure patients understand the information presented for the clinical study;
- Obtaining input from patient advisors on flexible options for follow-up visits and data collection techniques to reduce unnecessary burden on study/research participants who may have challenges fulfilling the follow-up schedule. Such techniques suggested may include allowing extended and/or weekend hours, permitting the study/research participants’ primary healthcare provider to perform some follow-up assessments, allowing phone or home visits by clinical researchers, allowing more convenient test labs to process routine bloodwork, or using mobile or online technologies to enable virtual or remote follow-up
- Working with patient advisors as needed during an ongoing study to discuss barriers to recruitment or other issues such as causes of study delays or challenges not anticipated before the study
- Discussing with patient advisors their views on which potential endpoints are meaningful in the treatment of the specific disease/condition;
- Working with patient advisors to inform the concepts that should be captured by patient-reported outcome (PRO) measures in the clinical study to better reflect outcomes that are important to patients;
- Working with patient advisors to inform the design of patient preference studies that may be used to inform the development of clinical studies or to help understand the benefit-risk trade offs among patients for the proposed treatment or multiple treatment options used for the disease/condition.
A concept model of the patient involvement roadmap is presented in the following figure, covering all the phases of a Medical Device lifecycle inspired by the phases of Medical Device Development and lifecycle management as presented in Figure 1 Lesson 1 and the EUPATI Patient Involvement in Medicine R&D roadmap, please see below:
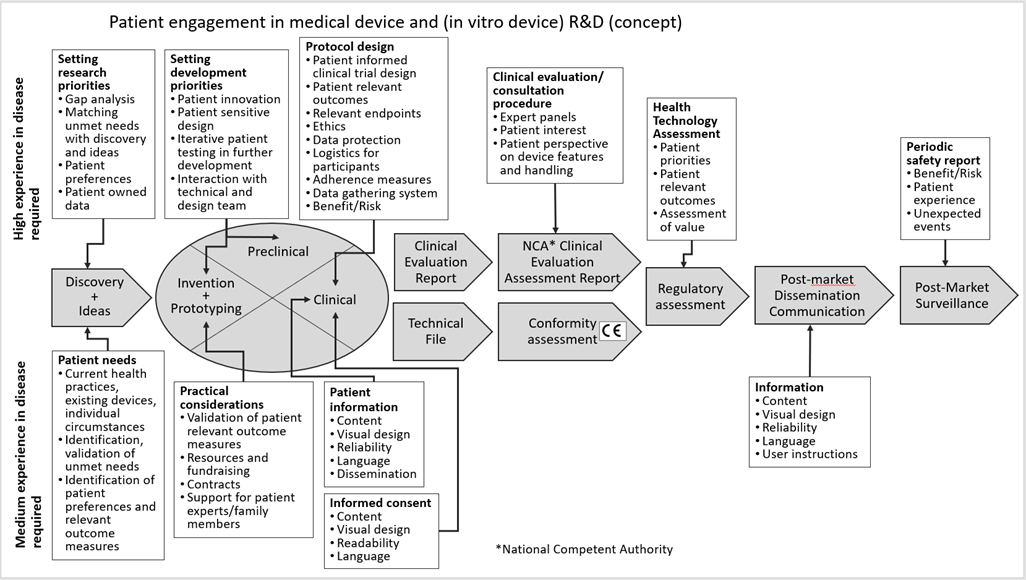
Figure 1: Concept roadmap of patient involvement in the different phases of the Medical Device R&D
Reference:
[1] Patient Engagement in the Design and Conduct of Medical Device Clinical Studies (fda.gov)