2. Levels of codes of conduct
Levels of codes of conduct
Professional associations and companies develop and implement codes of conduct at different levels which, irrespective of the level, remain consistent but are adapted to the respective environment (geographic, legislative, cultural etc.):
- At the global/international level. The International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) has developed a principles-based IFPMA Code of practice, including ethical principles (the IFPMA Ethos), covering interactions with HCPs, medical institutions and patient organisations, and the promotion of pharmaceutical products[1]. Many regional and local associations rely on the IFPMA Code as guidance for their own codes of conduct. In addition, codes of conduct of globally acting companies are also produced and have an international impact. They provide guidance for their affiliated national companies’ codes in any part of the world, of course adapted to local laws and regulations.
- At a regional level. The European Federation of Pharmaceutical Industries and Associations (EFPIA), the representative body of a large part of the pharmaceutical industry in Europe (36 national associations, 39 leading pharmaceutical companies and a growing number of small and medium-sized enterprises (SMEs)undertaking research and development in Europe of medicinal products and vaccines for human use), established the EFPIA Code of practice. As a member of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), the EFPIA Code embodies the principles of the IFPMA Code and Ethos. In the highly regulated environment of the research-based pharmaceutical industry in Europe the code reflects and, in some https://ifpma.org/publications/ifpma-code-of-practice-2019/parts, exceeds the requirements of Council Directive 2001/83/EC, as amended, relating to Medicinal Products, which, inter alia, directly deals with the promotion, information and advertising of medicinal products[2]. (See also Lesson 2 Advertising and promotion of medicines to the public and to HCPs.) Notably, EFPIA Member Companies must comply with any Applicable Codes and any laws and regulations to which they are subject.
- At a national level. Laws and regulations are usually implemented at a national level, (to note: in the EU a ‘regulation’ is immediate law applicable throughout the Union and should not be confused with the term ‘regulations’ as used in other context, and EU directives have to be transposed into national laws by content). In any case, national associations and company codes of conduct are developed and implemented to respect EU and national law and reflect country specific health system and cultural circumstances. By extension, where national associations are members of IFPMA and/or EFPIA they are held to accept the conditions of the IFPMA and EFPIA Codes and, subject to local laws and regulations, adopt codes that meet local requirements but are consistent with, and as comprehensive as, these Codes.
In all other territories, (i.e., where there are no local codes or appropriate laws and regulations, or where a company is not a member of a local/regional association), the IFPMA Code acts as a default code for the activities of companies that decide to voluntarily implement the IFPMA code.
In 2020 IFPMA ran a survey among IFPMA member associations. The Yes/No answers provided in this comparison simply indicate whether or not a certain provision is, or is not, covered by the relevant code in question as benchmarked to the global IFPMA Code of Practice. It provides a general overview of national and regional associations’ codes and their related provisions. This comparison table therefore is a snapshot of the global research-based pharmaceutical industry’s self-regulation mechanisms and the commitment of IFPMA members to the ethical promotion of medicines and interactions with healthcare professionals, patient organisations and medical institutions worldwide. The results of this Global Code Comparison can be accessed here.
A helicopter view of the interrelations between global/international, regional and national codes is given in the following figure (Fig. 1)
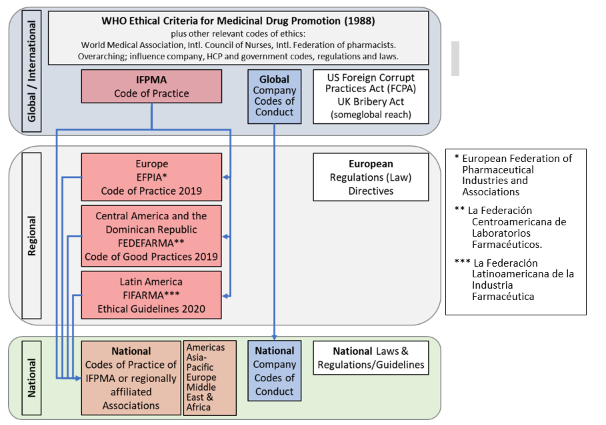
Figure 1: Summary schematic of different code and legislative/regulatory environments as they apply to international pharmaceutical companies. Arrows indicate direction of influence on next level codes which are adjusted to the respective environment (geographic, legislative, cultural etc.).
Adapted from: Francer et al. Philosophy, Ethics, and Humanities in Medicine 2014, 9:7 http://www.peh-med.com/content/9/1/7 and
IFPMA Global Code Comparison Report 2020, https://www.ifpma.org/resource-centre/ifpma-code-comparison-report/
[1] IFPMA Code of Practice (2019) Available at: IFPMA Code of Practice 2019 - IFPMA
[2] Directive 2001/83 consolidated Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02001L0083-20190726&from=EN