2. Principles of risk management
Principles of risk management
Marketing authorisations of medicines in the European Union (EU) are granted on the basis that a medicine’s benefits outweigh its risks for the target population in a given indication. Generally, no medicine will be without adverse reactions and these will vary in severity, likelihood of occurrence, effect on individual patients and public health impact. However, not all potential or actual adverse reactions will have been identified at the time of the initial marketing authorisation and some will only be discovered and characterised in the post-authorisation phase.
Consider for example that at the time of marketing authorisation data are usually available from Randomised controlled trials (RCT) obtained under defined conditions which do not represent real-life experience, for example:
- Participants in trials may not be exactly comparable to the overall population since they are selected according to a certain number of criteria
- Their exposure to new medicines may be different in terms of length of treatment and/or concomitant (accompanying) use of other medicines
- The number of patients exposed to the treatment during a RCT is usually limited. This means that potentially serious but infrequent events (idiosyncratic drug reactions – IDRs) may not have been detected during the RCTs.
The objective of risk management is to address uncertainties in the safety profile of a medicine at different points in its lifecycle and ensure that the balance of benefits and risks remains positive when a medicine is used in real world settings. Therefore, adequate plans need to be in place aiming at achieving these objectives.
Since the overall aim of risk management is to ensure that the benefits of a particular medicinal product exceed the risks and that this positive balance is maintained to the best possible degree over the lifetime of a medicine, risk management therefore involves the continuous process (circular and iteratively) of identifying, measuring and/or assessing risk, and developing and implementing strategies to manage it. This principle is shown in a schematic in the following figure (Fig. 1):
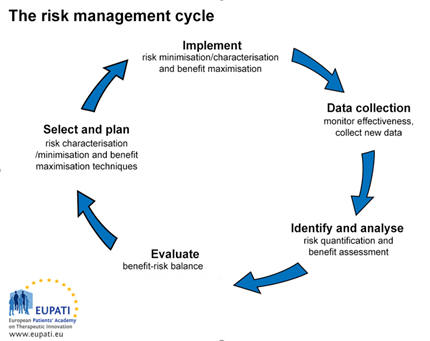
Figure 1: Schematic representation of the continuous risk management cycle throughout the lifetime of a medicinal product (from ‘Trilogy Writing & Consulting’).