4. Pharmaceutical Quality System (PQS)
Pharmaceutical Quality System (PQS)
Pharmaceutical companies are expected to have a robust quality system (PQS) in place to ensure that the quality of the medicinal products meet the requirements defined in the marketing authorisation, are safe and effective over their lifetime and as a result guarantee the safety of the patient.
The ICH Q10 guideline describes a comprehensive model for an effective quality management system for the pharmaceutical industry, referred to as the Pharmaceutical Quality System (PQS). It applies to the development and manufacture of pharmaceutical drug substances and medicines, including biotechnology and biological products, throughout the product lifecycle and has been adopted by regulators worldwide, including EMA and FDA. The pharmaceutical quality system “assures that the desired product quality is routinely met, suitable process performance is achieved, the set of controls are appropriate, improvement opportunities are identified and evaluated, and the body of knowledge is continually expanded.”(ICH Q10, Section 3.1.3 Commercial Manufacturing)
The following figure (Fig. 2), adapted from ICH gives an overview of the PQS model.
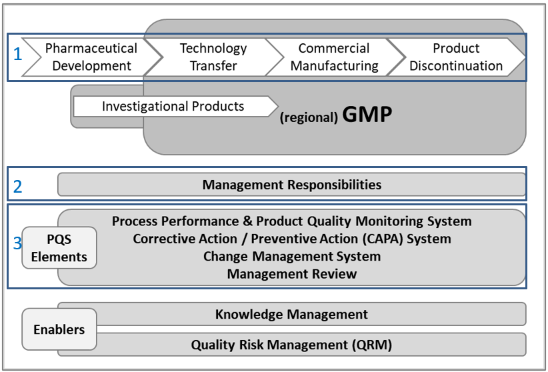
Figure 2: Overview of the ICH Q10 model of a PQS
The model is based on International Standards Organisation (ISO) quality concepts, includes applicable Good Manufacturing Practice (GMP) and complements ICH Q8 “Pharmaceutical Development” and ICH Q9 “Quality Risk Management”. It augments GMPs by describing specific quality system elements.
The objectives are to:
- Achieve product realisation
- Establish and maintain a state of control*
- Facilitate continual improvement
- Facilitate effective knowledge transfer and management
This is supported by so called ‘Enablers’, i.e., Knowledge & Quality Risk Management (QRM)
* A condition in which the set of controls consistently provides assurance of continued process performance and product quality.
The model is briefly described following the numbering as indicated in the model graph (Fig.2):
1) Lifecycle stages (in the context of ICH Q10)
a) Pharmaceutical Development (Goal – design a product and process that consistently delivers intended performance…)
i) Drug Substance development; Medicinal product formulation development
ii) Manufacturing process development and scale-up
iii) Analytical method development
b) Technology Transfer (Goal – transfer product and process knowledge to achieve realisation)
New product transfers during development through manufacturing transfers within or between manufacturing and testing sites for marketed products
c) Commercial Manufacturing (Goal – achieving realisation, establishing and maintaining a state of control and facilitating continual improvement)
i) Acquisition and control of materials
ii) Provision of facilities, utilities, and equipment
iii) Production (including packaging and labelling)
iv) Quality control and assurance (including release, storage, distribution)
d) Product Discontinuation (Goal – manage terminal stage of lifecycle effectively)
i) Continued product assessment and reporting
ii) Document archiving
iii) Sample retention
2) Management Responsibilities
c) Resource management
d) Internal communication
e) Management review
f) Oversight of outsourced activities
3) PQS Elements
a) Process performance and product quality monitoring system
A well-defined system for process performance and product quality monitoring to assure performance within a state of control and to identify improvement areas
b) Corrective action and preventive action (CAPA) system
a system for implementing corrective actions and preventive actions arising from the investigation of complaints, product rejections, non-conformances, recalls, deviations, audits, regulatory inspections and findings, and trends from process performance and product quality monitoring. A structured approach to the investigation process should be used, commensurate with the level of risk, in line with ICH Q9, with the objective of determining the root cause. CAPA methodology should result in product and process improvements and enhanced product and process understanding.
Of note: Corrective action is taken to prevent recurrence whereas preventive action is taken to prevent occurrence. (ISO 9000:2005)
c) Change management system
Innovation, the outputs of process performance and product quality monitoring and CAPA drive change. The change management system should ensure continual improvement is undertaken in a timely and effective manner. It should provide a high degree of assurance there are no unintended consequences of a change.
d) Management review of process performance and product quality
Management review should provide assurance that process performance and product quality are managed over the lifecycle. The management review system should identify appropriate actions and should support continual improvement.
ICH Q10 guideline on Pharmaceutical Quality System current version September 2015 EMA/CHMP/ICH/214732/2007
ICH Quality Guidelines cover various quality aspects in detail, such as the conduct of stability studies, defining relevant thresholds for impurities testing and a more flexible approach to pharmaceutical quality based on Good Manufacturing Practice (GMP) risk management.
WHO good manufacturing practices for pharmaceutical products: main principles