5.Ethical, legal and societal issues checklist
Ethical, legal and societal issues checklist
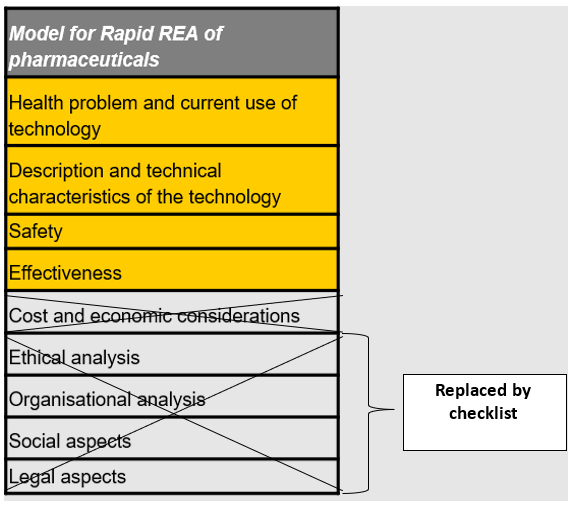
EUnetHTA developed the HTA Core Model which defines the content elements to be considered in a health technology assessment (HTA) and facilitates standardised reporting. Because different types of technology - such as pharmaceuticals, devices or procedures – may require different kinds of assessment a Core Model application for the rapid relative effectiveness assessment (REA) of pharmaceuticals, entitled the ‘HTA Core Model for Rapid Relative Effectiveness Assessment of Pharmaceuticals’[1] was developed. Instead of the nine domains included in the other applications of the Core Model, only the first four domains are included in the Model for Rapid REA of Pharmaceuticals (Health problem, Technical description of technology, Safety, Clinical effectiveness) (see table below). The ethical, organisational, societal and legal domains are replaced by a short checklist for quickly assessing the relevance of the ethical, organisational, societal and legal issues in the assessment – the ELSI checklist.
Table 1: Model for Rapid REA of pharmaceuticals, adapted from EUnetHTA Model for Rapid Relative Effectiveness Assessment of Pharmaceuticals
Below are the questions from the ELSI checklist from the ’HTA Core Model for Rapid Relative Effectiveness Assessment of Pharmaceuticals’. Pre-established problems/ issues, with regard to ethical, organisational, societal and legal aspects, that are common to the technology to be assessed and its comparator(s) will, as a rule, not be addressed, as it is not to be expected that the addition of a new pharmaceutical will lead to changes.
If a question is answered ‘yes’, further analysis may be warranted, otherwise the domain need not be further considered.
1. Ethical:
a. Does the introduction of the new medicine and its potential use/non-use instead of the defined, existing comparator(s) give rise to any new ethical issues?
b. Does comparing the new medicine to the defined, existing comparators point to any difference that may be ethically relevant?
2. Organisational:
a. Does the introduction of the new medicine and its potential use/non-use instead of the defined, existing comparators point to any differences that may be organisationally relevant?
b. Does comparing the new medicine to the defined, existing comparator(s) point to any differences that may be organisationally relevant?
3. Societal:
a. Does the introduction of the new medicine and its potential use/non-use instead of the defined existing comparator(s) give rise to any new societal issues?
b. Does comparing the new medicine to the defined, existing comparator(s) point to any differences that may be societally relevant?
4. Legal:
a. Does the introduction of the new medicine and its potential use/non-use instead of the defined, existing comparator(s) give rise to any legal issue?
b. Does comparing the new medicine to the defined, existing comparators point to any differences that may be legally relevant?
The Core Model says:
The domain of Organisational Aspects (abbreviation: ORG) considers the ways in which different kinds of resources (e.g. material artefacts, human skills and knowledge, money, attitudes, work culture) need to be mobilised and organised when implementing a technology, and the consequences they may further on produce in the organisation and the health care system as a whole. Organisational issues include e.g. work processes and patient/participant flow, quality and sustainability assurance, centralisation, communication and co-operation, managerial structure, and acceptance of a technology.