2. Outcomes: Which health effects matter?
Outcomes: Which health effects matter?
One of the challenges for HTA is that in the clinical trials conducted during development, the medicine may have been evaluated considering outcomes and endpoints that are not seen as relevant by HTA bodies/decision-makers. Regulatory assessment and HTA assessment consider different outcomes to be important. According to their remit regulatory authorities look at benefit-risk (efficacy) and HTA bodies look at ”added value” (effectiveness) regarding health technologies and outcomes. For example, pharmaceutical companies are required to submit evidence of beneficial health effects of a controlled trials as part of as part of the submission dossier to obtain regulatory approval. They must make choices when selecting an accepted comparator (often informed by early dialogue with regulatory authorities).
- A benefit of using technology, programme or intervention to treat a particular problem under ideal conditions – for example, in the context of research in a laboratory or a rigorous protocol for a randomizes clinical trial. EMA: The measurement of a medicine’s desired effect under ideal conditions, such as in a clinical trial.
- Efficacy: A benefit of using technology, programme or intervention to treat a particular problem under ideal conditions – for example, in the context of research in a laboratory or a rigorous protocol for a randomizes clinical trial. EMA: The measurement of a medicine’s desired effect under ideal conditions, such as in a clinical trial.
- Effectiveness: The benefit of using a technology, programme or intervention to address specific problem under general or routine conditions, rather than under controlled conditions, for example, by a physician in a hospital or by a patient at home.
- Treatment Effect: The effect of the subjects’ health status or well-being attributable only to a treatment or intervention. Note: investigators seek to estimate the true effect of a treatment of intervention by calculating the difference between the outcome obtained in the experimental group and the control group.
Conversely, an HTA body might be evaluating if a newly approved medicine would show positive outcomes for patients in a clinical setting and provide additional value to existing therapies (not necessarily strictly pharmaceutical) and, in addition, justifying a possibly higher cost to the health system. No treatment is available, against placebo, from randomised controlled trials as part of the submission dossier to obtain regulatory approval. They must make choices when selecting an accepted comparator (often informed by early dialogue with regulatory authorities). Conversely, an HTA body might be evaluating if a newly approved medicine would show positive outcomes for patients in a clinical setting and provide additional value to existing therapies (not necessarily strictly pharmaceutical) and, in addition, justifying a possibly higher cost to the health system.
Differences between regulatory approval, HTA and patients regarding health technologies can be seen in the table below.
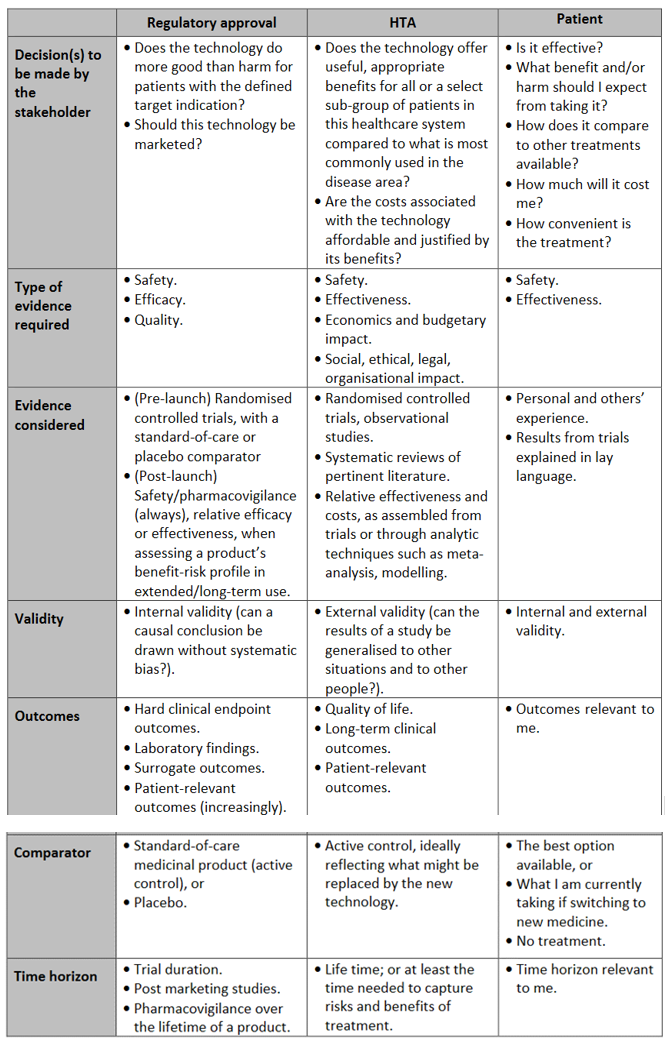
Table 1
Adapted from: 1. Tsoi B, Masucci L, Campbell K, Drummond M, O’Reilly D, Goeree R. Harmonization of reimbursement and regulatory approval processes: a systematic review of international experiences. Expert Rev Pharmacoecon Outcomes Res. 2013 Aug;13(4):497–511. 2. Henshall C, Mardhani-Bayne L, Frønsdal KB, Klemp M. Interactions between health technology assessment, coverage, and regulatory processes: emerging issues, goals, and opportunities. Int J Technol Assess Health Care. 2011 Jul;27(3): 253–60.