6. Non-Clinical Safety Testing
Step 6. Non-Clinical Safety Testing
Is it safe to proceed to clinical testing? The next stage in the development process involves safety testing in animals, which is governed by specific rules and regulations of Good Laboratory Practice (GLP). No candidate compound can become an investigational medicinal product (IMP) to be tested in humans (in "clinical studies") before its safety profile has been established in animal safety studies. Medicines development is tightly controlled. The law imposes rules and regulations about what is done and how it is done.
Before the non-clinical testing work can be done, larger amounts of the candidate compound need to be produced so that all the appropriate tests can be carried out. This manufacturing process also has to follow strict guidelines and regulations called Good Manufacturing Practice (GMP).

Figure 6. Animal studies are part of the non-clinical studies.
The regulations state which studies have to be done and which type of animals have to be used to obtain relevant information. These include looking at effects:
- in the animal overall
- in all the animal tissues and organs (systemic toxicology studies)
- on the ability of the animals to reproduce and develop normally (reproductive toxicology studies)
- on the skin or eyes (local toxicology studies)
- any allergies (hypersensitivity studies)
- on the chromosomes and genes (genotoxicity studies)
- any effects on cancer generation (carcinogenicity studies)
These studies are shown in the diagram below
.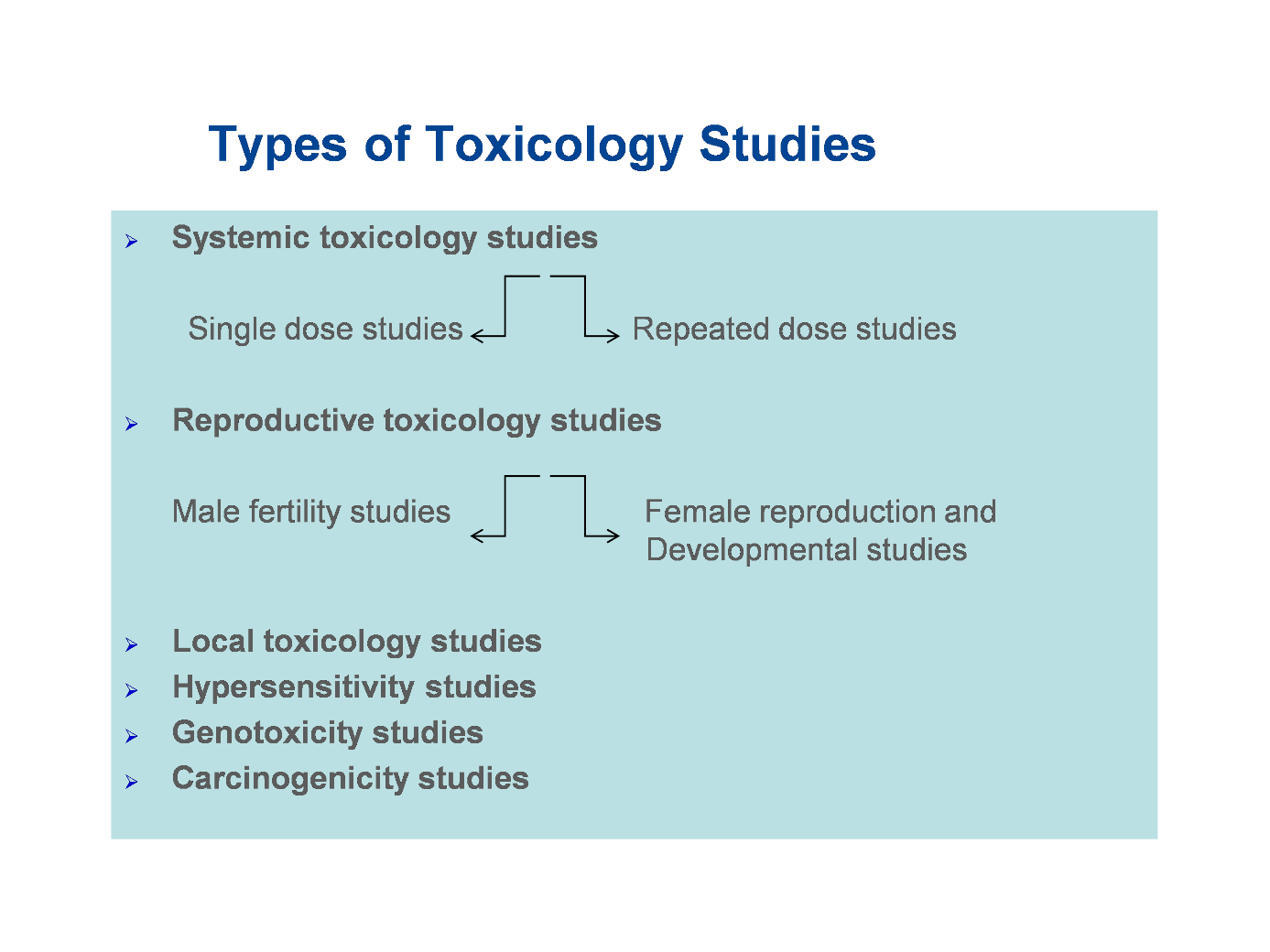
Figure 7: Types of toxicology (nonclinical safety) studies.
These studies not only show the safety profile in animals, but also provide important information about:
- How the substance enters the body (Absorption)
- Distribution around the body
- Breakdown of the substance by the body (Metabolism)
- How the substance leaves the body (Excretion).
This is usually abbreviated to 'ADME'.
All of this information is used to decide if the candidate compound can proceed into the first human (clinical) study and if so, what doses to use.
Summary: To continue into clinical testing, with humans, the candidate compound must have shown an acceptable safety profile in all the necessary non-clinical toxicology studies. However, not all of the non-clinical safety studies will have been completed. For example, long-term carcinogenicity studies take on average two years and continue at the same time as the clinical trials.