4. Clinical effectiveness assessment in HTA
1. Clinical effectiveness assessment in HTA
1.2. Asking relevant questions
When assessing the clinical effectiveness of a new medicine, the HTA body must carefully consider all outcomes associated with it. It is important to know about these outcomes in order to ask the relevant questions about the medicine’s effectiveness.
There is increasing understanding that the outcomes that may seem relevant to clinicians are not always those considered most important by patients. For this reason, it is advisable for patients to be involved in designing studies in order to make sure that information is collected on the outcomes that matter to them. For instance, in recent years, it has been recognised that quality of life is an important outcome for patients. This has led to the development of specific methodologies to create quality-of-life measures and so-called ‘patientreported outcomes’ (PRO) within in clinical trials and pharmacoepidemiologic studies .
One approach to ensure that all the important outcomes of a particular technology are examined is to use an analytic framework – for instance, following a flowchart. Analytic frameworks are helpful to visualise all of the outcomes associated with an intervention, and to highlight where there are uncertainties.
This is illustrated in the analytic framework shown in Figure 1:
- Cause and effect are depicted by arrows (curved arrows indicate harmful outcomes);
- Health improvement outcomes (such as decreased mortality) are depicted by rectangles (sharp cornered rectangles show clinically relevant endpoints - those that are perceived by the patient, such as reduced chest pain. Round cornered rectangles show intermediate endpoints, including surrogate endpoints, which cannot be perceived by the patient, such as cholesterol level in blood).
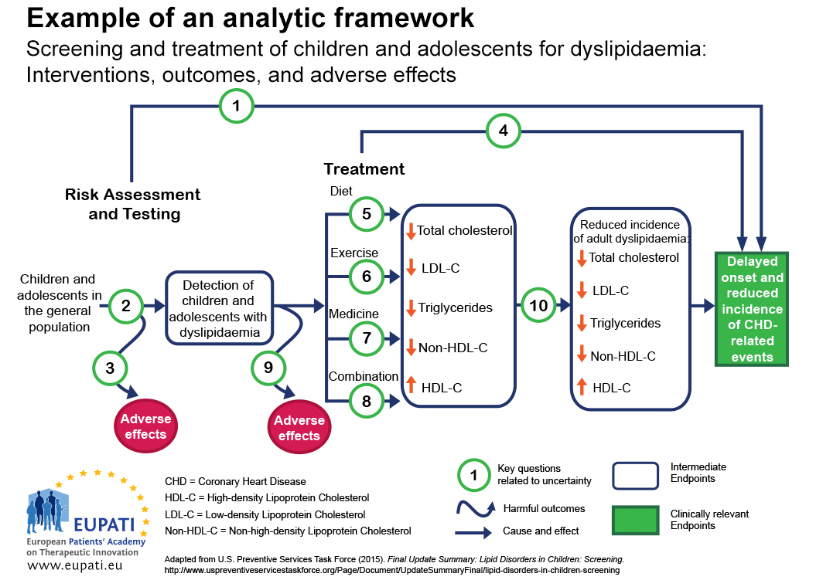
Figure 1: Template for an analytic framework. (Source: AHRW) (1)
Key question 1. Is screening for dyslipidaemia in children/adolescents effective in delaying the onset and reducing the incidence of CHD (coronary heart disease) related events?
Key question 2. What is the accuracy of screening for dyslipidaemia in identifying children/adolescents at increased risk of CHD-related events and other outcomes?
Key question 3. What are the adverse effects of screening (including false positives, false negatives)?
Key question 4. In children and adolescents, what is the effectiveness of medicine, diet, exercise, and combination therapy in reducing the incidence of adult dyslipidemia, and delaying the onset and reducing the incidence of CHD-related events and other outcomes (including optimal age for initiation of treatment)?
Key questions 5-8. What is the effectiveness of medicine, diet, exercise, and combination therapy for treating dyslipidemia in children/adolescents (including the incremental benefit of treating dyslipidemia in childhood)?
Key question 9. What are the adverse effects of medicine, diet, exercise, and combination therapy in children/adolescents?
Key question 10. Does improving dyslipidemia in childhood reduce the risk of dyslipidemia in adulthood?
Key question 11 (not pictured). What are the cost issues involved in screening for dyslipidemia in asymptomatic children?
In general, an assessment of clinical effectiveness should address the following questions:
1. How comprehensive was the information provided for a thorough HTA? provided for a thorough HTA?
- Systematic reviews conducted in a rigorous and transparent manner with extensive information provided by pharmaceutical companies are most likely to be comprehensive and balanced;
- Real world studies should still consider whether what is observed is consistent with previous studies (2).
2. How accurate is the
information?
- Real-world information gathered within the health system for which a decision has to be made is most likely to be relevant;
- Relying solely on reviews or summaries without a clear understanding of the methods used may not be balanced.
3. Is anything missing?
- Not involving patients or providers to help understand outcomes of importance, not conducting a thorough search or soliciting extensive information from pharmaceutical companies may lead to missing information.
4. How understandable is the information?
- Information that considers changes meaningful to patients should be interpreted carefully and correctly and presented in a way understandable for lay persons.