1. Introduction
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
It is important for a rational development of medicines that researchers ask relevant questions and answer them with appropriate studies. Clinical studies can be classified based on when the studies occur during clinical development (Phase I, II, III, IV) or why they are done (human pharmacology, therapeutic exploratory, therapeutic confirmatory, therapeutic use).
Before any clinical trial is carried out, results of non-clinical studies should be acceptable and show that the medicine is safe for the proposed human testing. The safety of participants in the first human studies (first-in-human studies) is the most important consideration. Non-clinical testing might find potential risk factors for investigational medicinal products (IMP). However, the ability of non-clinical studies to predict safety issues in humans may be limited because the nature of the target is more specific to humans or because of other factors. Attention should be given to the estimation of the initial dose to be used in humans and when the dose is escalated (increased).
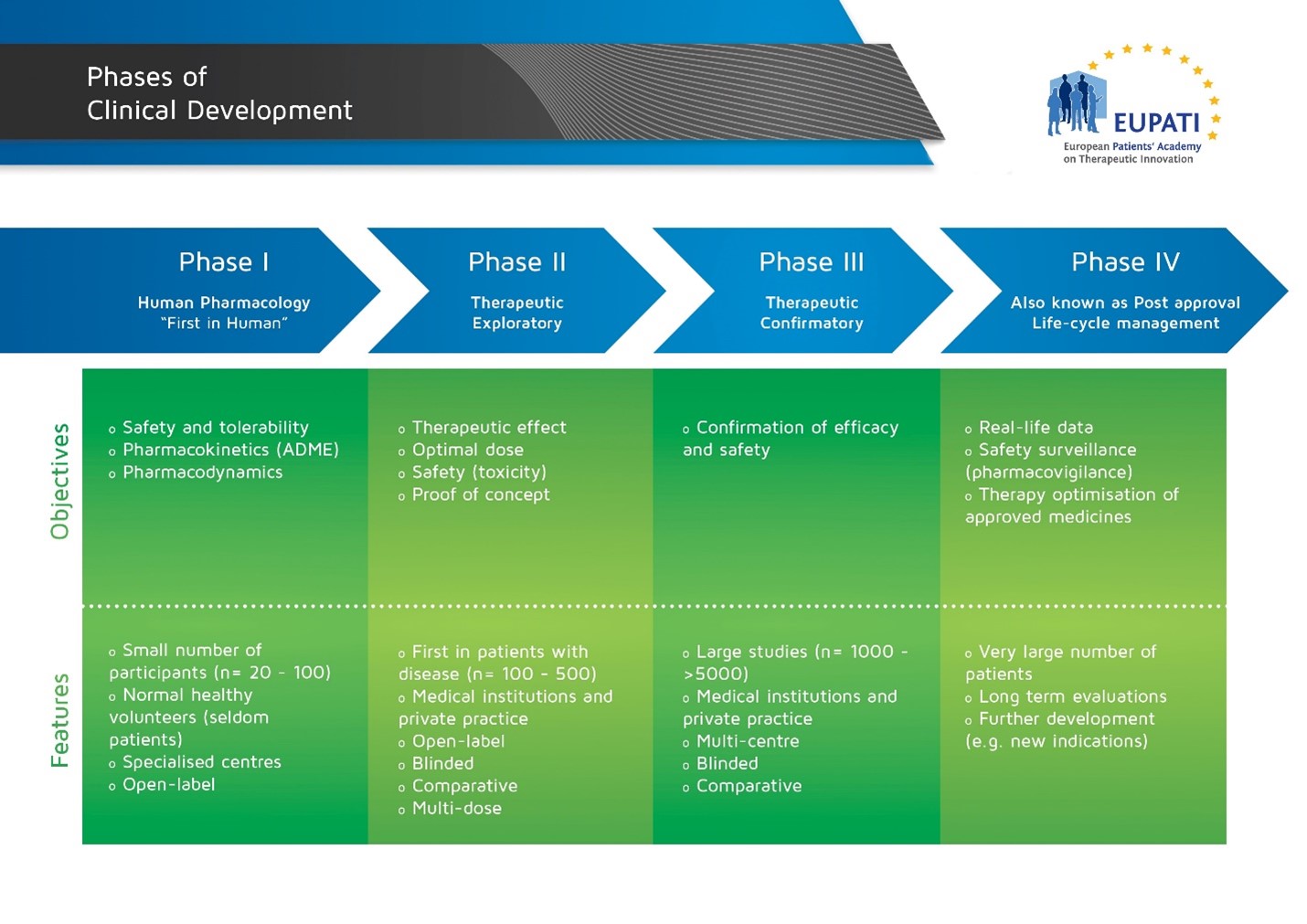 Image with details of the four phases of Clinical Development.
Image with details of the four phases of Clinical Development.
Studies are executed in phases in the development of a medicinal product so that the results of prior studies can influence the plan of later studies. However, the phases do not mean that the order of studies is fixed. Data that come forth during development will often prompt a change of the development strategy. New data may also suggest the need for additional studies that are typically part of an earlier phase. Below are a couple of examples.
Human pharmacology studies are usually done in Phase I but many Phase I studies are also carried out in phase II, III and IV (e.g. blood level data in a late study may suggest the need for a medicine-medicine interaction study, or adverse effects may suggest the need for further dose finding).
Phase I trials normally initiate clinical medicine development. However, a study may combine several phases with different fundamental objectives. Therefore, studies are often labeled not just as phase I, for instance, but alternatively: early Phase I (IA) or late Phase I (IB), or perhaps Phase I/II, early Phase II (IIA) or late Phase II (IIB), or Phase II/III, etc.
There are key questions that must be answered by the studies in early clinical development (Phases I & II):
Phase I
-
Is the medicine safe in humans? At what levels? (Tolerance)
-
What does the body do to the medicine? (Pharmacokinetics (PK))
-
What does the medicine do to the body? (Pharmacodynamics (PD))
-
What interactions are there? (with other medicines, substances, food and drink)
Phase II
- Is the medicine safe in patients?
- What does the medicine do to the body? (Pharmacodynamics (PD))
- Does the medicine seem to work in patients? At what dose? (Effect)