Completion requirements
View
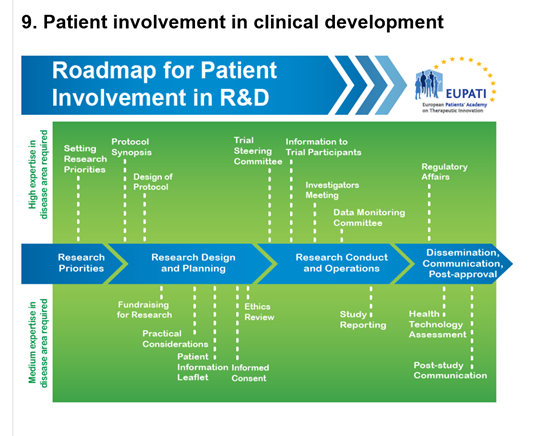
9. Patient Involvement in the Informed Consent Process
Patient representatives can work with the trial organisers to design the informed consent. They can help ensure that the informed consent documentation:
- Is written entirely in understandable and non-technical or scientific language.
- Does not contain persuasive language.
- Explains that participation in the study is entirely voluntary.
- Provides fair perspectives on the possible disadvantages and risks of participation.
- Outlines any direct benefits for the individual and any other beneficial outcomes of the study, including furthering our understanding of the research topic.