Completion requirements
View
Examples of HTA bodies in Europe and other tasks
Table 2: Sample HTA bodies structure and funding arrangements
| Example HTA bodies | Year | Funder | Decision-Maker |
|---|---|---|---|
| 1. Basque Office for Health Technology Assessment, Spain |
1992 | Public | Regional |
| 2. Hayes, Inc. USA |
1989 | Private | Various |
| 3. Swedish Council on Technology Assessment in Health Care (SBU), Sweden |
1987 | Public | National-Regional |
| 4. National Institute for Health and Care Excellence (NICE) |
1999 | Public | National – regional England, Scotland and Wales |
|
5. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (Institute for Quality and Efficiency in Healthcare) (IQWiG), Germany |
2004 | Public | Federal |
| 6. Haute Autorité de Santé (HAS), France | 2004 | Public | Federal |
Some examples of HTA bodies in Europe include:
- France - Haute Autorité de Santé (HAS) - http://www.has-sante.fr
- Germany - IQWIG (Institute for Quality and Efficiency in Health Care) - https://www.iqwig.de/en/
- Scotland - Scottish Medicines Consortium (SMC) - www.scottishmedicines.org.uk/Home
- Sweden - Tandvårds- och Läkemedelsförmånsverket (TLV) - https://www.tlv.se/in-english.html
- United Kingdom – National Institute for Health and Care Excellence (NICE) - https://www.nice.org.uk/
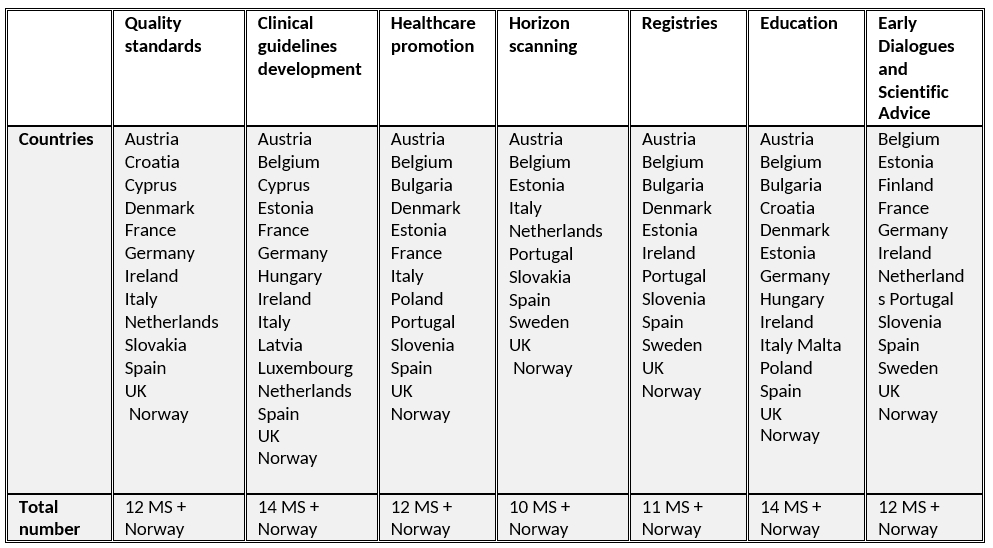
In addition to their main role of producing HTA recommendations a number of HTA bodies in the EU and Norway perform at least one of the other tasks as shown in an overview in the following table, adapted from the EC study 2018.
Table 3. Other tasks performed by national HTA bodies