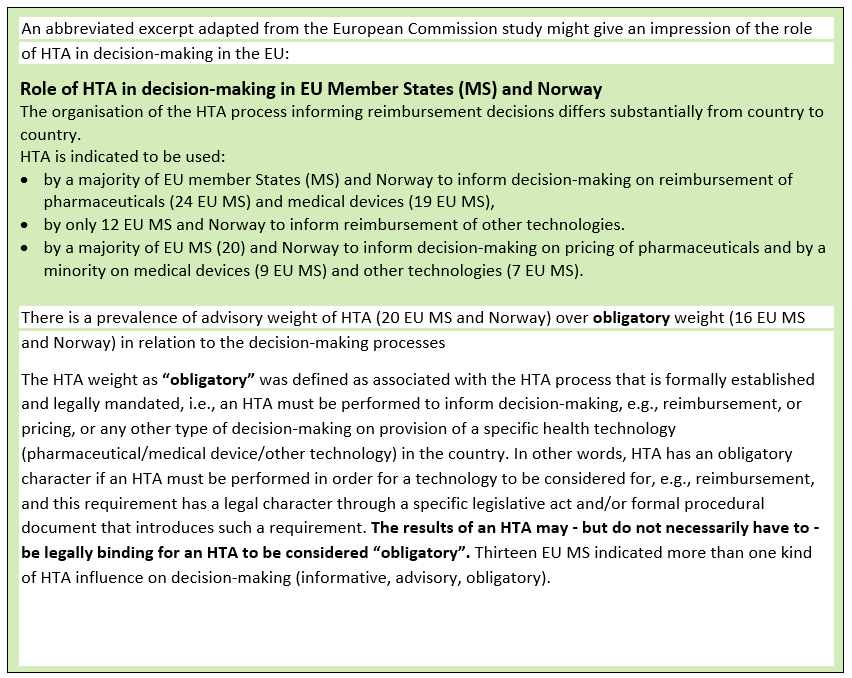
HTA and decision-making
An HTA body’s role within a healthcare system is context-specific, and the nature of an HTA body’s output is likely to reflect societal values. Once a technology has been assessed, a (social) process follows in which decisions about resource allocation and access to technology have to be made. These are often contentious issues such as in the use of an abortion pill, or cannabis-based medicines.
There are two main components of HTA: Assessment and Appraisal/Decision making. In some countries, the assessment and appraisal/decision functions of an HTA may be carried out by separate bodies:
- One body may be dedicated to an assessment function – effectively collecting and synthesising evidence or critically reviewing evidence submissions (you will read more about synthesising in Course 3 Lesson 1.)
- Another, different body (e.g. a commission installed for this purpose) may be dedicated to an appraisal and decision function - considering the assessment in light of wider factors related to the local context.
Note that in Germany, the evaluation component of HTA is carried out by IQWIG (Institute for Quality and Efficiency in Health Care) and the appraisal component and decision-making is carried out by the GBA (Gemeinsamer Bundesausschuss, Federal Joint Committee). Also note that in some European countries there is a specific HTA body that deals with medicines, whereas in some countries the HTA body covers both medicines and other interventions such as devices, surgical procedures and (in some cases) public health interventions.