Completion requirements
View
3. Randomised Controlled Clinical Trial Designs
3.4. Cross-over Trial Design
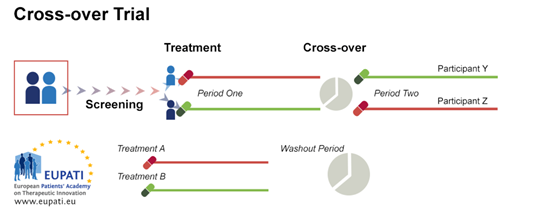
Cross-over randomisation is when participants receive a sequence of different treatments (for example, the candidate compound in the first phase and the comparator/control in the second phase). Each treatment starts at an equivalent point, and each p
serves as their own control. It presents some advantages, such as low variance due to treatment and control being the same participant, and the possibility of including a number of treatments. There must be a washout period between treatments – this
is a period where the participant does not use any treatment. Participants must have a stable and chronic disease. Cross-over trials require fewer participants but for a longer period of time.
Advantages
- Low variance due to participant and control being the same.
- Can include a number of treatments.
Disadvantages
- Requires a long-term illness as treatments are applied one after the other.
- Carry over effects need to be avoided (washout period must be sufficiently long).