10. Combined advanced-therapy medicinal products (combined ATMPs)
These medicines contain an advanced therapy plus one or more medical devices – for example, cells that are fixed in a matrix or structure to engineer or reconstruct tissue.
The EMA defines combined ATMPs as: “Combined ATMPs incorporate a cellular part consisting of cells or tissues and one or more medical devices or one or more active implantable medical devices as an integral part of the product”.
An example of a combined ATMP is the ‘autologous chondrocyte implantation’ (ACI) to repair joint cartilage (Figure 8). Patient cells (autologous chondrocytes) are grown in the laboratory until the required number of cells is obtained. The cells are planted onto a collagen membrane which is implanted into the damaged cartilage. The cells repair the damaged tissue, while the medical device (the collagen membrane) keeps the cells in place.
Preparation of
bioengineered chondrocyte cell sheets
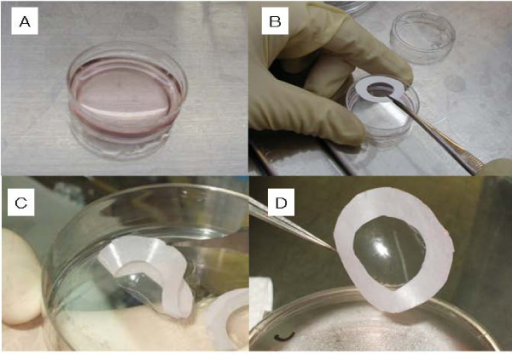
Figure 8: Preparation of bioengineered chondrocyte cell sheets for cartilage repair. A: dish containing growing cells. B: a membrane is placed on the sheet of cells and used to lift the cells out of the dish (C and D). Image courtesy of Mitani G et al., created under Creative Commons licence CC BY 2.0 http://openi.nlm.nih.gov/detailedresult.php?img=2662823_1472-6750-9-17-1&req=4