2. Pre-submission contact with regulatory agencies
Various activities, directly involving regulators, in preparation of a marketing authorisation application (MAA) can and should take place. A brief overview is given in the following graph (adapted from an EMA presentation) with an approximate timeline in which such activities may occur.
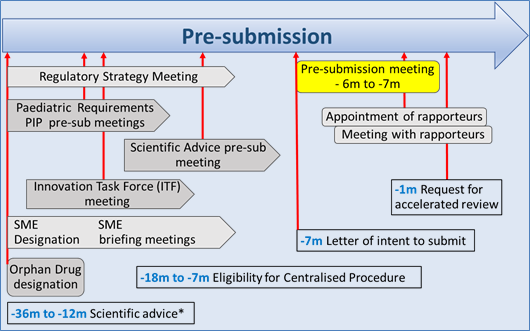
Figure 1: The approximate timelines of activities before a submission are denoted as ‘minus months’ using the notation
*Of note: Scientific advice in various forms is available throughout the development of a medicine (usually not for free) and not only in a relatively short time frame before submission of a MAA.
Of particular importance is the Pre-submission meeting with EMA staff on request by the company, in order to obtain further information and guidance. It is strongly recommended by EMA, even for experienced users of the centralised procedure.
Such a meeting will normally take place 6 to 7 months before the submission date.
The pre-submission meeting are organised to:
- Discuss legal, regulatory and scientific issues
- Discuss final practical & regulatory aspects of upcoming application
- Clarify application-specific issues not addressed on the EMA website
- Ensure that application will meet all requirements for validation
- Reconfirm various administrative/procedural/legal issues; requirements may have changed
|
Moreover, the project team of the pharmaceutical company will meet with the EMA team who will be involved when the application is received including the potential rapporteurs. |
|
In case of applications under the mutual recognition procedure (MRP), decentralised (DCP) or national procedure (NP), pre-submission contacts are also possible with the relevant NCA(s) and are equally useful.
Answers to many questions in relation to applications via the centralised procedure may be found on the EMA website, shown below.
Having obtained all the information needed, the company can finalise the application dossier.