1. Pharmacoepidemiology
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
For medicines it provides an estimate of the probability of beneficial and adverse effects in a population. It can be called a bridge science spanning both clinical pharmacology and epidemiology. Pharmacoepidemiology concentrates on clinical patient outcomes from therapeutic interventions by using methods of epidemiology and applying them to understand the determinants of beneficial and adverse effects, effects of genetic variation on medicine effect, duration-response relationships, clinical effects of medicine-drug interactions, and the effects of medication non-adherence. Pharmacoepidemiology also involves the conduct and evaluation of public programmes to improve medication use on a population basis.
Although the main contributions to pharmacoepidemiology come from epidemiology and clinical pharmacology, other disciplines, like medicine, biostatistics, computer science and administrative databases inform pharmacoepidemiology as well.
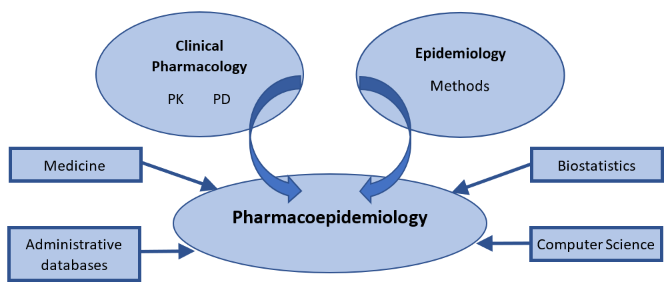
Figure 1.: Illustration
of the relationship between pharmacoepidemiology and several contributing disciplines.
PK: pharmacokinetics; PD: pharmacodynamics.
Pharmacovigilance (or postmarketing surveillance) (see Epidemiology/pharmacoepidemiology
an integral part of Pharmacovigilance) is
an important part of pharmacoepidemiology that involves continuous monitoring
in a population for unwanted effects and other safety concerns arising in medicines
that are already on the market. In addition, without pharmacovigilance, there would
be limited information about the effectiveness of medicines in practice such as
how a medicinal product is used, whether people continue to take it, and what
outcomes are associated with it in diverse patient populations. Remember that
efficacy refers to whether the medicinal product can produce a specific
therapeutic outcome in a controlled environment. This is investigated in
clinical trials. In the “real world,” effectiveness needs to be considered. In
other words, can the medicinal product produce the desired therapeutic outcome
in practice where the environment is not controlled and is it sufficiently safe?
You can read more about efficacy and effectiveness in Health
Technology Assessment module, in the course HTA
and Evaluation Methos: Quantitative.
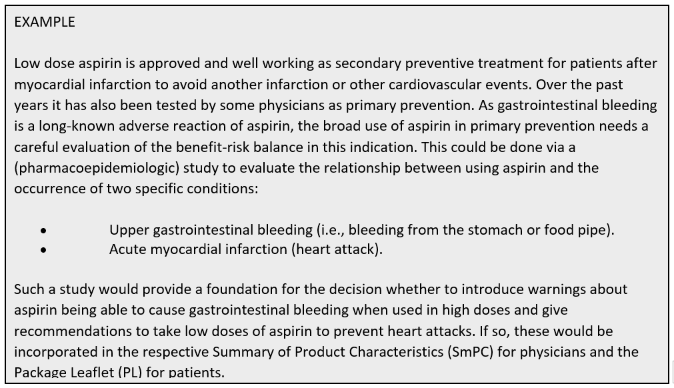
Since pharmacoepidemiology studies provide information about medicines utilisation and safety, these studies are important throughout the entire medicines’ life cycle. Therefore, Pharmacoepidemiology starts from discovery research and continues through pre-marketing research and into post-approval monitoring and safety studies (Figure 2). This applies as well to vaccines and medical devices.
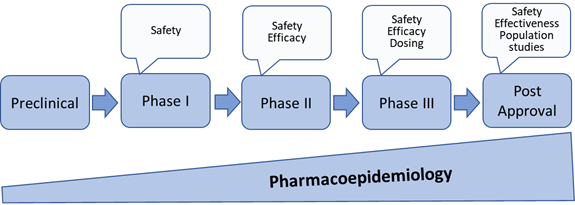
Figure 2: Pharmacoepidemiology through medicines development and post approval[1]
[1] Upadhyay Srishti *, Shrivastava Saumya, Kumar Deepak, Kabra Atul, Baghel Uttam Singh, Pharmacoepidemiology: A Review, Asian Journal of Pharmaceutical Research and Development.2019; 7(2):83-87 DOI: http://dx.doi.org/10.22270/ajprd.v7i2.464