Completion requirements
View
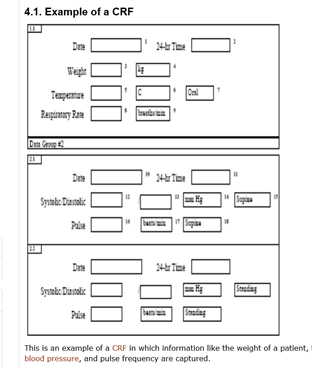
3. What is a Case Report Form (CRF)?
The ICH-GCP Guideline glossary defines a ‘case report form’ (CRF) as:
‘A printed, optical, or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial participant's.'