1. Bioavailability
1.6. Example 3 - relative bioavailability
What you have to consider is that Brian has trouble breathing, so he needs the API to reach the target site in the body as fast as possible.
The target site for asthma medication is the lungs. Let’s forget everything about side effects for a minute and whether Brian prefers to receive an injection or taking a tablet. We will instead focus only on bioavailability. We have already talked about absolute bioavailability when we compared the AUC for an IV injection versus a tablet. Now we want to compare two formulations containing the same API, but administered via different routes.
Let’s calculate the relative bioavailability of an inhalator versus a tablet as was suggested to Brian in Example 3. The AUC for the inhalation is 20 mg h/L while the AUC for the tablet is 60 mg h/L. The dose given for inhalation is 1 mg whereas the tablet contains 5 mg. What is the relative bioavailability of the inhalation versus the tablet? And what would you recommend Brian to choose?
To calculate the relative bioavailability the following equation (relative bioavailability equation) must be used.
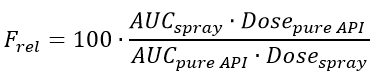
Then, the values given in Example 3 are inserted to the equation.
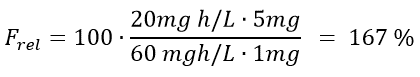
The result is that the absolute bioavailability of the inhalation compared to the tablet is 167%. You can therefore conclude that for this example, more API from the inhalation spray reaches the target site compared to the tablet. This is most likely due to a high degree of first pass metabolism of the substance after it is given orally. Therefore, Brian should be advised to use the inhalator. Another advantage of using the inhalator is that it will administer the active substance locally because the lungs are the target site. A tablet, on the other hand, needs to be dissolved in the gastro intestinal tract before uptake can take place. This would increase the risk of side effects because the API will be present in the systemic circulation. When an API is administered locally, the amount of it that reaches systemic circulation is minimized and so is the risk of systemic side effects.