1. Bioavailability
1.1. Intravenous bioavailability
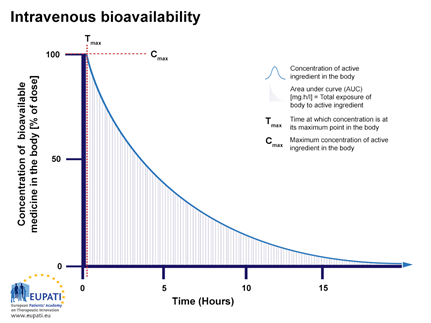
Figure 1: The percentage of active substance in the body or bioavailability, after direct injection into the blood stream and observed over a period of 15 hours. The area under the curve (AUC), is shaded. Tmax is the time when the highest concentration of the medicine is found in the blood, Cmax is the maximum concentration.
After injection, an API reaches the target site after a complex journey in the bloodstream and body.Immediately after injection, the bioavailability will be 100% (seen on the y-axis in Figure 1 as Cmax and on the x-axis at Tmax). So, if 75 milligram (mg) of active substance is injected into the bloodstream, then 100% corresponds to 75 mg active substance. The concentration of the API in the body will then decrease over time (Figure 1) because, a fraction of it will be metabolised or excreted.