4. Special cases in marketing authorisations: Advanced therapy medicines (ATMPs)
| Site: | EUPATI Open Classroom |
| Course: | Regulatory procedures- Marketing-Authorisations and their lifecycle management |
| Book: | 4. Special cases in marketing authorisations: Advanced therapy medicines (ATMPs) |
| Printed by: | Guest user |
| Date: | Friday, 4 July 2025, 4:50 AM |
1. Advanced therapy medicines (ATMPs)
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
Advances in science have yielded a new class of innovative and complex biological medicinal products based on manipulation[1] of genes, somatic cells or tissue engineering, so-called advanced therapy medicinal products (ATMP). They are very different from medicines based on chemical entities or of biological / biotechnological origin due to their complexity. While these novel health technologies have the potential to address unmet medical needs in the treatment of disease as well as the repair of tissue or organ defects, they also involve specific risks (e.g., immunogenicity, viral safety, tumorigenicity etc.) that need to be accounted for in an appropriate regulatory framework.
Moreover, the regulatory context for cell or gene-based therapies was heterogeneous across EU Member States until the early 2000s and resulted in divergent national procedures for classification and authorisation. In order to harmonise, and as per the EC “to ensure the free movement of ATMPs within Europe, to facilitate access to the EU market, and to foster the competitiveness of European companies in the field while guaranteeing the health protection for patients”, the European Council adopted a lex specialis[2] in the form of the regulation on advanced therapy medicinal products (Regulation (EC) No 1394/2007), (in force 30 December 2008) to be read in conjunction with Directive 2001/83/EC and Regulation (EC) No 726/2004 as amended.
The ‘ATMP Regulation’ lays down specific rules for the authorisation, supervision and pharmacovigilance of ATMPs and establishes the Committee for Advanced Therapies (CAT) at the EMA.
[1] More than “minimally manipulated”: For structural tissue: processing that alters the original relevant characteristics of the tissue relating to the tissue’s utility for reconstruction, repair, or replacement. For cells or non-structural tissues: processing that alters the relevant biological characteristics of cells or tissues.
[2] The Latin `lex specialis' notion comes from the legal maxim `lex specialis derogate legi generali'. A `lex specialis' is a `law' which governs a specific subject matter. The legal maxim means that a law governing a specific subject matter overrides a law that only governs general matters. For our subject, Regulation (EC) No. 1394/2007 overrides the general EU pharmaceutical legislation, which applies unless otherwise specified in the ATMP regulation.
1.1. Criteria and classification
In this regulatory framework ATMPs are defined and classified into the following types according to criteria established in the relevant legislation:
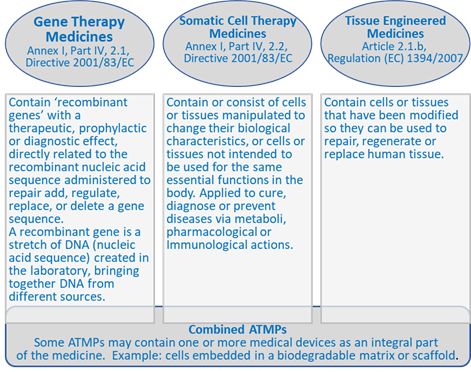
Figure 3
The regulatory framework for ATMPs further encompasses the following elements (adapted from the EMA):
- the centralised marketing authorisation procedure (see lesson 1 this course) which is obligatory for ATMPs. However, according to recital 7, the ATMP Regulation does not affect decisions of individual Member States about the use of embryonic stem cells or animal cells which may still be covered by national legislation.
- the CAT: an EMA multidisciplinary expert Committee (Committee for Advanced Therapies).
Main task: to assess advanced therapy products and prepare a draft opinion before the CHMP adopts a final opinion and the authorisation is granted by the Commission
Other tasks with participation of the CAT:- classification of ATMPs: The CAT delivers scientific recommendations on ATMP classification after consultation with the European Commission.
- certifying quality and non-clinical data for small and medium-sized enterprises (SMEs) developing ATMPs;
- contributionto scientific advice, in cooperation with the Scientific Advice Working Party (SAWP);
- delivery of advice on the conduct of efficacy follow-up, pharmacovigilance or risk-management systems for ATMPs;
- advice to the CHMP on any medicine that may require expertise in ATMPs for the evaluation of its quality, safety or efficacy;
- scientific assistance in developing any documents relating to the objectives of the Regulation on ATMPs;
- provision of scientific expertise and advice for any Community initiative related to the development of innovative medicines and therapies requiring expertise on ATMPs;
- support to the work programmes of the CHMP working parties.
- technical requirements adapted to the particular characteristics of these products
1.2. Incentives
The ATMP Regulation also introduced a number of incentives for manufacturers of advanced therapies, particularly small and medium-sized enterprises (SME). The aim is foremost to provide regulatory certainty to various questions relating to R&D of ATMPs in pursuing a marketing authorisation. These include:
- Scientific recommendation on advanced therapy classification (“ATMP classification”);
- Companies can consult the EMA on a voluntary basis to determine whether a medicine they are developing is an ATMP.
- ‘What guidelines are applicable to my product?’
- 60-day procedure (often shorter),
- NNo fee
- All classification outcomes are published (summary)
- For early developments (no expectation that the product already has substantial non-clinical or clinical data available);
- Certification of (early) quality and (early) non-clinical data for SMEs only
- Questions on Quality, Non-clinical and clinical development. According to Article 18 of Regulation (EC) No 1394/20071 which provides that SMEs developing an ATMP may submit to the EMA all relevant quality and, where available, non-clinical data
- ‘Is my product development so far on track for a future Marketing Authorisation Application?’
- 90-day procedure
- Applicant will always receive the evaluation report and List of issues for future consideration
- If positive evaluation: Certificate by EMA;
- Scientific advice:
- A fee reduction of 90% for SMEs and 65% for other applicants on any scientific advice given with respect to ATMPs;
- Scientific advice on the design and conduct of Pharmacovigilance and risk management systems;
- Reduction of fees for marketing authorization (50%) for hospitals and SMEs if a public health interest in the ATMP can be proven; furthermore, the 50% fee reduction is also granted for post-authorisation activities in the first year following the granting of the product’s marketing authorisation.
Of note: in case where the reference medicinal product is a biologic (or ATMP), the general criteria for generic MAAs do not apply; rather, follow-on biologicals that are similar to the reference product must still provide a certain amount of preclinical and clinical data in support of its MAA. For further details on biosimilars see EMA-Biosimilars.
Of note: The ATMP Regulation, in line with Directive 2001/83/EC, applies to ATMPs manufactured by industrial methods and intended to be marketed in EU Member States. Under the so-called hospital exemption, certain ATMPs are excluded from its provisions (Article 3(7) of Directive 2001/83/EC 2001), if they are:
- prepared on a non-routine basis according to specific quality standards,
- used within the same Member State in a hospital under the exclusive professional responsibility of a medical practitioner,
- to be compliant with an individual medical prescription for a custom-made product for an individual patient.
ATMPs falling under the above exemptions do not need to apply for a MA, but can rather be authorised for use by the concerned Member State if they apply with national requirements for quality, traceability and pharmacovigilance, equivalent to those for ATMPs with a centralised MA. This non-routine application of ATMPs was intended to facilitate the research and development of advanced therapies by academic groups, hospitals or not-for-profit organisations.
Legal basis:
Regulation 1394/2007/EC on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004 – i.e. the provisions of the ATMP Regulation are now also reflected in those acts.
Guidelines with specific ATMP relevance
GMP for ATMPs
EC adopted Guidelines on Good Manufacturing Practice (GMP) specific for ATMPs (November 2017). The Guidelines provide a specific GMP framework that is adapted to the specific characteristics of ATMPs. Guidelines on Good Manufacturing Practices (GMP)
GCP for ATMPs
EC adopted Guidelines on Good Clinical Practice (GCP) specific for ATMPs (October 2019). Guidelines on Good Clinical Practice (GCP) specific for Advanced Therapy Medicinal Products (ATMP)
GMO (Genetically Modified Organism) requirements for investigational products Questions & Answers document
Further reading: European Commission-DG Health and Food Safety and European Medicines Agency Action Plan on ATMPs (europa.eu)