1. Non-standard marketing authorisations
1. Non-standard marketing authorisations
1.3. Differences between conditional marketing authorisations and marketing authorisations under exceptional circumstances
Conditional Marketing Authorisations (CMA) are distinct from marketing authorisations granted under exceptional circumstances. In the case of the CMA, the authorisation is not intended to remain conditional indefinitely. Instead, once the missing data are provided, it should be possible to convert it into a standard marketing authorisation, not subject to specific obligations. A marketing authorisation under exceptional circumstances should not be granted when a CMA is more appropriate. For example, a CMA is granted in the absence of comprehensive clinical data when it is likely that the applicant will be capable of providing such data in a foreseeable timeframe. Though, the fulfilment of any specific obligations imposed as part of the marketing authorisation under exceptional circumstances, is aimed at the provision of information on the safe and effective use of the product, it will normally not lead to the completion of a full dossier.
The following table (Table 1) gives a short summary of the differences between conditional MA and MA under exceptional circumstances. Figure 2 indicatively shows the relation between a standard MA, conditional MA and MA under exceptional circumstances.
Table 1: Differences between conditional MA and MA under exceptional circumstances
|
Conditional marketing authorization |
Marketing authorization under exceptional circumstances |
|
Authorisation before availability of comprehensive data. Comprehensive data to be generated post-authorisation in agreed timelines. |
Authorisation when comprehensive data on efficacy and safety cannot be provided, but it is still appropriate to grant the authorisation due to exceptional circumstances. |
|
Medicines without comprehensive data belonging to at least one of the following categories:
and fulfilling all of the following criteria: |
Medicinal products without comprehensive data on the efficacy and safety under normal conditions of use, because:
|
|
Authorisation valid for one year, on a renewable basis based on reconfirmation of positive benefit- risk balance. |
Authorisation initially valid for 5 years (renewable), but status of fulfilment of specific obligations and the impact of the specific obligations’ data on the benefit / risk balance to be reassessed annually. |
|
Once the comprehensive data are provided, it can become a “standard” or “normal” marketing authorisation. |
Will normally not lead to completion of a full dossier and become a “standard” marketing authorisation. |
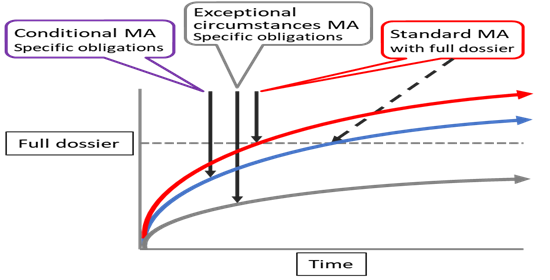
Figure 2: Indicative relation between standard MA, CMA and MA under exceptional circumstances (not to scale). Black arrows indicate time of approval of MA, the broken line transition from CMA to standard MA after providing comprehensive data (Full dossier). Curved lines depict accumulation of data for MA (comprehensive data set; Full dossier) and beyond, over time. To note: On average, a CMA is converted into a standard MA within 4 years (data from EMA). Both, CMA and MA under exceptional circumstances can avail of ‘accelerated assessment’.