1. Non-standard marketing authorisations
| Site: | EUPATI Open Classroom |
| Course: | Regulatory procedures- Marketing-Authorisations and their lifecycle management |
| Book: | 1. Non-standard marketing authorisations |
| Printed by: | Guest user |
| Date: | Tuesday, 8 July 2025, 3:03 PM |
1. Non-standard marketing authorisations
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
The development of new approaches to foster access to novel treatments for patients has continued since its earliest introduction and continues today. This should happen at the earliest appropriate time in the product life-span in a sustainable fashion. Regulators have been actively pursuing various approaches, recognising that patients and providers are willing to tolerate greater risks, especially risks of the unknown about medicines, when the morbidity of the disease is significant or when the disease is potentially life-threatening.
In the EU, mechanisms providing faster patient access to new medicines include ‘approval under exceptional circumstances’ (1993) and ‘conditional marketing authorisations’ (2005) for obtaining authorisations subject to certain annually reviewable conditions. Further, in order to meet, in particular, the legitimate expectations of patients and to take account of the increasingly rapid progress of science and therapies, ‘accelerated assessment procedures’ have been set up, reserved for medicines of major therapeutic interest.
A short recap: For an approved standard 'full' MA, all required quality, non-clinical and clinical data have been provided, and the benefit/risk profile is considered positive by the regulatory authorities, i.e., that quality, safety and efficacy of the product are established. Irrespective of that, the MA for the medicine may be granted subject to specific conditions (e.g., to conduct post-authorisation safety and/or efficacy studies[1], or the existence of an adequate pharmacovigilance system). A full MA is valid for five years and may then be renewed on the basis of a re-evaluation of the benefit/risk balance and shall then be valid for an unlimited period (with exceptions on grounds related to pharmacovigilance)[2].
Legal basis: Marketing authorisation subject to conditions Article 9.4 b, c, ca, cb, cc. of Regulation (EC) No 726/2004 and Article 21a of Directive 2001/83/EC
In the following the mentioned procedures for earlier access will be described in more detail.
[1] A post-authorisation efficacy study (PAES), imposed in accordance with Regulation (EC) No 357/2014, or a post-authorisation safety study (PASS) in accordance with Article 1(15) of Directive 2001/83/EC.
1.1. Conditional Marketing Authorisation (CMA)
For certain categories of medicines, marketing authorisations may be granted on the basis of less complete data than what is normally required, in order to meet unmet medical needs* (as defined within the Article 4 (2) Commission Regulations EC n 507/2006) of patients and in the interest of public health*. In such cases, it is possible for the EMA Committee for Medicinal Products for Human Use (CHMP) to recommend the granting of a marketing authorisation subject to certain specific obligations to be reviewed annually ('conditional marketing authorisation').
*Quote from guideline: “there is no single definition of what constitutes major public health interest. This should be justified by the applicant and assessed by the CHMP on a case-by-case basis. Typically, the justification could present the arguments to support the claim that the medicine addresses to a significant extent the unmet medical needs for maintaining and improving the health of the Community, for example, by introducing new methods of therapy or improves existing ones. It is noted that a new mechanism of action or a technical innovation per se may not necessarily represent a valid argument for justifying major interest from the point of view of public health.”
Medicines that are eligible for centralised approval by the Commission and which:
- aim at the treatment, the prevention or the medical diagnosis of seriously debilitating diseases or life-threatening diseases; or
- are also intended to be used in emergency situations, in response to public threats duly recognised either by the WHO or by the Community for a public health emergency (e.g., a pandemic; see also 1.1.1. Rolling review); or
- are designated as orphan medicinal products in accordance with the Orphan Regulation.
Criteria
A conditional MA may be granted where although comprehensive clinical data referring to the safety and efficacy of the medicine have not been supplied, all the following requirements are met:
- The currently known benefit-risk balance of the medicine is positive.
- It is likely that the comprehensive clinical data will be provided by the applicant in the future.
- Unmet medical needs will be fulfilled.
- The benefit to public health of the immediate availability of the medicine outweighs the risk that additional data are still required.
* ‘unmet medical needs’ means a condition for which there exists no satisfactory method of diagnosis, prevention or treatment authorised in the Community or, even if such a method exists, in relation to which the medicinal product concerned will be of major therapeutic advantage to those affected’ (Art. 4(2) of Regulation (EC) No 507/2006)
Conditions
Conditional marketing authorisations are valid for one year and can be renewed annually.
Once a conditional marketing authorisation has been granted, the marketing authorisation holder (MAH) must fulfil specific obligations within defined timelines. These obligations could include:
- completing ongoing or to conduct new studies or
- collecting additional data to confirm the medicine's benefit-risk balance remains positive
- specific obligations in relation to the collection of pharmacovigilance data
Of note: Specific obligations generally include clinical studies and exceptionally, in the context of public health emergencies, studies to provide further assurance on the pharmaceutical quality of e.g., vaccines.
The marketing authorisation can be converted into a standard marketing authorisation (no longer subject to specific obligations) once the marketing authorisation holder fulfils the obligations imposed and the complete data confirm that the medicine's benefits continue to outweigh its risks. Initially, this is valid for 5 years. It can then be renewed for unlimited validity.
As for any medicine, if new data show that the medicine’s benefits no longer outweigh its risks, EMA can take regulatory action, such as suspending or revoking the marketing authorisation. EMA can also take regulatory action if the company does not comply with the imposed obligations.
Report on 10 years of experience at the EMA avaliable at: Conditional marketing authorisation - Report on ten years of experience at the EMA (europa.eu)
Of note: A conditional marketing authorisation is different from an emergency use authorisation, which some countries use to permit the temporary use of an unauthorised medicine in an emergency situation. An emergency use authorisation is not a marketing authorisation.
Legal basis:
Article 14(7) of Regulation (EC) No 726/2004
Regulation (EC) No 507/2006
CHMP guideline on the scientific application and the practical arrangements necessary to implement Commission Regulation (EC) No 507/2006 on the conditional marketing authorisation for medicinal products for human use falling within the scope of Regulation (EC) No 726/2004.
1.1.1 Rolling review
A rolling review is a regulatory process that EMA uses to speed up the assessment of a promising medicine during a public health emergency. The EMA rolling review can accelerate every step of the regulatory pathway while ensuring that robust evidence on efficacy, safety and quality is generated to support scientific and regulatory decisions.
Normally, all data on a medicine or vaccine’s effectiveness, safety and quality and all required documents must be ready at the start of the evaluation for a formal marketing authorisation application (MAA). In the case of a rolling review, EMA’s CHMP reviews data as they become available from ongoing studies and can come to an opinion on the marketing authorisation sooner. Once the CHMP decides that sufficient data are available, the company can submit a formal MAA. EMA then has the option of recommending a conditional marketing authorisation. EU legislation foresees that conditional marketing authorisation is used as the fast-track authorisation procedure during public health emergencies to speed up approval and save lives.
A prime example for this approach is the Corona virus pandemic as described in the following.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus that causes COVID-19 (coronavirus disease 2019), the respiratory illness responsible for the COVID-19 pandemic. First identified in the city of Wuhan, Hubei, China, the World Health Organization declared the outbreak a Public Health Emergency of International Concern on 30 January 2020, and a pandemic on 11 March 2020. Epidemiological studies estimate that each infection results in an average of 2.39 to 3.44 new ones when no members of the community are immune and no preventive measures are taken.
The virus primarily spreads between people through close contact and via aerosols and respiratory droplets that are exhaled when talking, breathing, or otherwise exhaling, as well as those produced from coughs or sneezes. (adapted from WIKIPEDIA)
The EMA plays an important role in enabling the development, scientific evaluation, approval and monitoring of COVID-19 vaccines in the European Union (EU). EMA has adopted the rolling review procedure under its EMA emerging health threats plan as one measure to expedite the regulatory process and the availability of safe and efficacious vaccines against COVID-19.
The rolling review process in short:
- EMA's scientific committees – CHMP and Pharmacovigilance Risk Assessment Committee (PRAC)- review data as they become available on an ongoing basis.
- This review is done with the support of the COVID-19 EMA pandemic Task Force (COVID-ETF).
- The review takes place while development is still ongoing.
- The COVID-ETF has to agree on the start of a rolling review.
- There can be several rolling review cycles during the evaluation of one product while data continue to emerge. The number of cycles depends on the amount of data to be assessed.
- Each cycle is pre-agreed between EMA and the applicant.
- The submission for each cycle is done in eCTD (electronic Common Technical Document) format.
- In addition to the newly available data, each Rolling Review submission occurs in eCTD format with an application form, a CTD-Module 2 overview and responses to a cumulative listing of all outstanding questions from previous review cycles.
- During the rolling review, EMA assesses whether the data package is complete enough to invite the applicant to submit a formal marketing authorisation application.
End of rolling review - EMA processes the application under a shortened timetable.
- After review and upon positive recommendation by EMA the European Commission grants an EU-wide marketing authorisation
- National Competent Authorities decide on introduction of the newly approved vaccine and vaccination policies.
- Other data must be provided by the company, after approval (e.g., long-term protection data)
Figure 1, adapted from EMA, shows a graphic view of the rolling review process for COVID-19 vaccines:
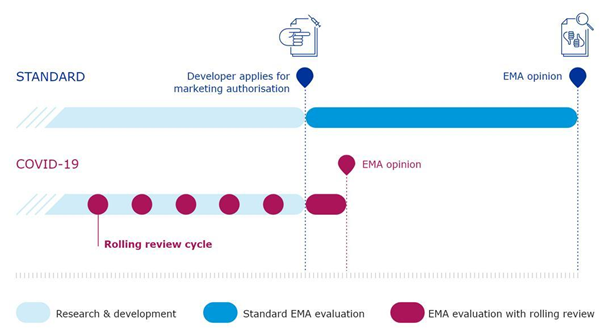
Figure 1: Graphic representation of the rolling review process for COVID-19 vaccines as deployed by EMA. The shortened time until the EMA opinion on the MAA is clearly visible.
Further reading: EMA initiatives for acceleration of development support and evaluation procedures for COVID-19 treatments and vaccines (16 September 2021 EMA/213341/2020 Rev.3)
EMA COVID-19 vaccines: development, evaluation, approval and monitoring
EMA COVID-19 guidance: evaluation and marketing authorisation
1.2. Marketing Authorisation under exceptional circumstances
An authorisation under exceptional circumstances is possible for products for which the applicant can demonstrate in the application that it is not possible to provide comprehensive data on the efficacy and safety under normal conditions of use for objective, verifiable reasons. Consequently, it will normally hardly ever be possible to assemble a full dossier in respect of a marketing authorisation granted under exceptional circumstances.
Depending on the application the different options are:
- the indications for which the product in question is intended are encountered so rarely that the applicant cannot reasonably be expected to provide comprehensive evidence; or
- in the present state of scientific knowledge, comprehensive information cannot be provided; or
- it would be contrary to generally accepted principles of medical ethics to collect such information.
Conditions
A marketing authorisation may be granted subject to certain specific obligations. These obligations may include the following:
- completion of an identified programme of studies within a time period specified by the competent authority, the results of which will form the basis of a reassessment of the benefit/risk profile,
- supply of the medicine on prescription only and in certain cases administration only under strict medical supervision, possibly in a hospital,
- the package leaflet and any medical information has to mention that a marketing authorisation has been granted subject to certain specific obligations to be reviewed annually,
- a requirement for the applicant to introduce specific procedures, in particular concerning the safety of the medicine,
- notification to the competent authorities of any incident relating to the use of the medicine, and action to be taken.
The fulfilment of specific procedures/obligations is aimed at the provision of information on the safe and effective use of the product.
This type of authorisation is reviewed annually to re-assess the benefit-risk profile.
The renewal of the marketing authorisation of a medicine under exceptional circumstances follows the same rules as a “normal” marketing authorisation. After 5 years, the marketing authorisation will then be renewed under exceptional circumstances for an unlimited period, unless the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal.
Legal basis:
Article 14 (8) of Regulation (EC) No 726/2004
Guideline on procedures for the granting of a marketing authorisation under exceptional circumstances, pursuant to Article 14 (8) of Regulation (EC) No 726/2004.
Article 22, Directive 2001/83/EC, as amended
Annex I, Part II, documentation for applications in exceptional circumstances, Directive 2001/83/EC, as amended.
1.3. Differences between conditional marketing authorisations and marketing authorisations under exceptional circumstances
Conditional Marketing Authorisations (CMA) are distinct from marketing authorisations granted under exceptional circumstances. In the case of the CMA, the authorisation is not intended to remain conditional indefinitely. Instead, once the missing data are provided, it should be possible to convert it into a standard marketing authorisation, not subject to specific obligations. A marketing authorisation under exceptional circumstances should not be granted when a CMA is more appropriate. For example, a CMA is granted in the absence of comprehensive clinical data when it is likely that the applicant will be capable of providing such data in a foreseeable timeframe. Though, the fulfilment of any specific obligations imposed as part of the marketing authorisation under exceptional circumstances, is aimed at the provision of information on the safe and effective use of the product, it will normally not lead to the completion of a full dossier.
The following table (Table 1) gives a short summary of the differences between conditional MA and MA under exceptional circumstances. Figure 2 indicatively shows the relation between a standard MA, conditional MA and MA under exceptional circumstances.
Table 1: Differences between conditional MA and MA under exceptional circumstances
|
Conditional marketing authorization |
Marketing authorization under exceptional circumstances |
|
Authorisation before availability of comprehensive data. Comprehensive data to be generated post-authorisation in agreed timelines. |
Authorisation when comprehensive data on efficacy and safety cannot be provided, but it is still appropriate to grant the authorisation due to exceptional circumstances. |
|
Medicines without comprehensive data belonging to at least one of the following categories:
and fulfilling all of the following criteria: |
Medicinal products without comprehensive data on the efficacy and safety under normal conditions of use, because:
|
|
Authorisation valid for one year, on a renewable basis based on reconfirmation of positive benefit- risk balance. |
Authorisation initially valid for 5 years (renewable), but status of fulfilment of specific obligations and the impact of the specific obligations’ data on the benefit / risk balance to be reassessed annually. |
|
Once the comprehensive data are provided, it can become a “standard” or “normal” marketing authorisation. |
Will normally not lead to completion of a full dossier and become a “standard” marketing authorisation. |
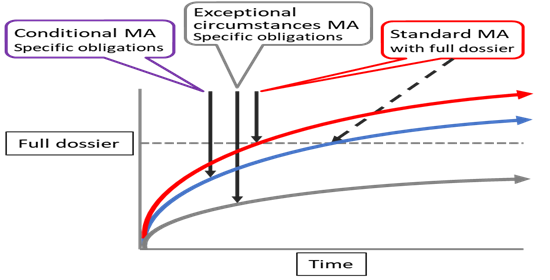
Figure 2: Indicative relation between standard MA, CMA and MA under exceptional circumstances (not to scale). Black arrows indicate time of approval of MA, the broken line transition from CMA to standard MA after providing comprehensive data (Full dossier). Curved lines depict accumulation of data for MA (comprehensive data set; Full dossier) and beyond, over time. To note: On average, a CMA is converted into a standard MA within 4 years (data from EMA). Both, CMA and MA under exceptional circumstances can avail of ‘accelerated assessment’.