5. European Public Assessment Report (EPAR)
| Site: | EUPATI Open Classroom |
| Course: | Product information and information to the public |
| Book: | 5. European Public Assessment Report (EPAR) |
| Printed by: | Guest user |
| Date: | Saturday, 5 July 2025, 8:39 PM |
1. European Public Assessment Report (EPAR)
A European public assessment report (EPAR) is published for every medicine application that has been granted or refused a marketing authorisation. This follows Council regulation (EC) 726/2004 Art. 13 (3) which stipulates: ‘The Agency (EMA) shall immediately publish the assessment report on the medicinal product for human use drawn up by the Committee for Medicinal Products for Human Use and the reasons for its opinion in favour of granting authorisation, after deletion of any information of a commercially confidential nature. The European Public Assessment Report (EPAR) shall include a summary written in a manner that is understandable to the public. The summary shall contain in particular a section relating to the conditions of use of the medicinal product’.
An EPAR provides public information on a medicine, including how it was assessed by EMA. The EPAR reflects the scientific conclusions of the relevant EMA committee at the end of the assessment process, providing the grounds for the committee opinion on whether or not to approve an application. For products following the Central authorisation procedure of medicines (CAPs) the EMA will provide an EPAR.
EPARs can be found using a search function at: Medicines | European Medicines Agency (europa.eu)
The following figure (Fig. 3), a screenshot of the medicines search page, shows the EMA webpage where, applying the search function, EPARS can be located and gives an example of the layout and search fields (filters) the user can apply to refine the search according to the information sought:
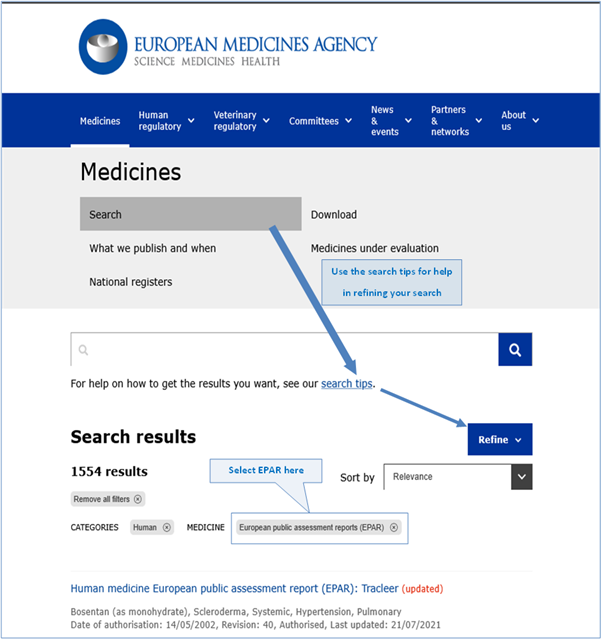
Figure 3: Screenshot, truncated, of the EMA webpage for accessing EPARs.
The Heads of medicines (HMA) ‘Mutual Recognition Index’ or ‘MRI Product Index’ includes medicines approved in the Member States of the EU according to the Mutual Recognition (MRP) or Decentralised Procedure (DCP), called Nationally Approved Products (NAP). The respective Reference Member State (RMS), provides a Public Assessment Report (PAR) which is similar to an EPAR in that it follows Directive 2001/83 EC Art. 21(4) for NAPs: ‘The national competent authorities shall make the assessment report publicly accessible without delay, together with the reasons for their opinion, after deletion of any information of a commercially confidential nature. The public assessment report shall include a summary written in a manner that is understandable to the public. The summary shall contain, in particular, a section relating to the conditions of use of the medicinal product.
PARs can be found through any of the following:
Heads of Medicines Agencies: MRI Product Index
MRI - Home
MRI - Product search - if the user looks for a specific medicine
MRI - Document search - a list of NCA Websites with links to their information on Authorised Human Medicines if available
A screenshot of the entry page from the first URL above is shown in the following figure (Fig. 4)
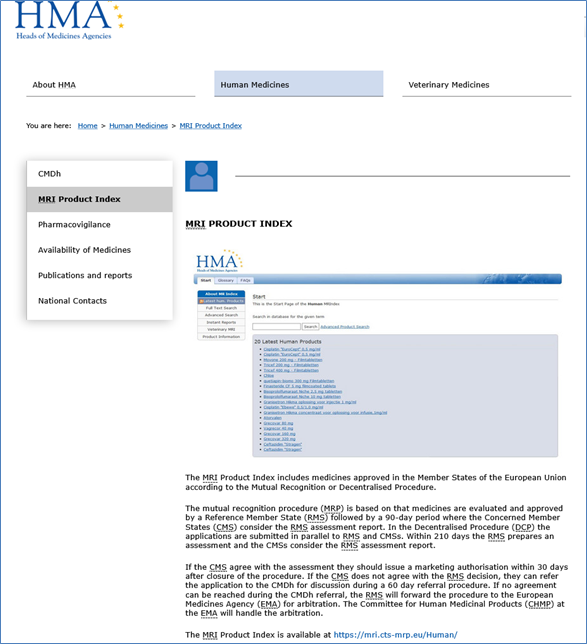
Figure 4: Screenshot, truncated, of the HMA web-start page for the MRI product index.
1.1. EPAR Structure and content
An EPAR is not a single document but consists of several information components, including a core set of regulatory documents. EPARs are shown on the EMA website and the individual components can be viewed online, downloaded and/or printed.
However, some of the information is regarded as confidential and is not included, like detailed information on the manufacturing of a medicine.
EPARs are updated periodically to reflect the latest regulatory information on medicines. If the original terms and conditions of a marketing authorisation are varied, e.g. through changes in the benefit-risk balance, a new indication of an already authorised medicine, the EPAR and consequently SmPC, Labelling and PL are updated to reflect such changes.
The below figure (Fig. 5) provides an exemplary overview of the structure and content of an EPAR for ‘Brintellix’, displayed on the EMA website using four different sections containing different components of the EPAR.
To note: The detailed format and content of the public-friendly overview have been adapted and enhanced over time, but the main principles are set out in a reflection paper: Reflection paper on European public assessment report summary for the public.
Figure 5: An example of the EPAR for the medicine ‘Brintellix’ taken and adapted from the EMA website, with content notes on the four components.
https://www.ema.europa.eu/en/medicines/human/EPAR/brintellix