6. Direct healthcare professional communication. Communicating medicines safety information directly to healthcare professionals: an introduction to DHPC
| Site: | EUPATI Open Classroom |
| Course: | Pharmacovigilance - Risk management |
| Book: | 6. Direct healthcare professional communication. Communicating medicines safety information directly to healthcare professionals: an introduction to DHPC |
| Printed by: | Guest user |
| Date: | Sunday, 8 June 2025, 5:57 AM |
1. Introduction
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
The European Medicines Agency (EMA) and European
Union (EU) member states regulatory authorities
share the responsibility to communicate safety information on medicinal products after their
marketing authorisation to patients and healthcare professionals. As healthcare
professionals play an essential role in disseminating safety information, direct healthcare professional communication (DHPC) is the primary way of communicating ‘new or emerging
safety information’.
2. What is a direct healthcare professional communication (DHPC)?
The EMA produced the ‘Guideline on good pharmacovigilance Practices (GVP) Module XV – Safety communication’. This document details also the preparation, coordination and dissemination of DHPCs.
As defined by the EMA, a direct healthcare professional communication or DHPC is ’a communication intervention by which important safety information is delivered directly to individual healthcare professionals by a marketing authorisation holder (MAH) or a competent authority, to inform them of the need to take certain actions or adapt their practices in relation to a medicinal product.
The document notes also that ‘DHPCs are not replies to enquiries from healthcare professionals, nor are they meant as educational material for routine risk minimisation activities’.
3. When are DHPCs disseminated?
A DHPC should be disseminated when there is a need to take immediate action, or change current practice, in relation to a medicinal product. The EMA guideline provides details about the different situations where a DHPC is necessary or should be considered. As specified in the EMA guideline, situations when a DHPC may become necessary are the following:
- a suspension, withdrawal or revocation of an MA for safety reasons;
- an important change to the use of a medicine due to the restriction of an indication, a new contraindication, or a change in the recommended dose due to safety reasons;
- a restriction in availability or discontinuation of a medicine with potential detrimental effects on patient care.
Other situations where dissemination of a DHPC should be considered are:
- new major warnings or precautions for use in the product information;
- new data identifying a previously unknown risk or a change in the frequency or severity of a known adverse reaction;
- new evidence that the medicinal product is not as effective as previously considered;
- new recommendations for preventing or treating adverse reactions or to avoid misuse or medication error with the medicinal product;
- ongoing assessment of an important potential risk, for which data available at a particular point in time are insufficient to take regulatory action.
4. The seven steps of a DHPC
The seven steps of a DHPC
The general process of a DHPC can be described in seven steps which are followed by EMA and the national competent authority (NCA):
- Request or initiative: The EMA or an NCA can request a DHPC. The MAH may also take the initiative to disseminate a DHPC. In both cases, the MAH should seek the agreement of the relevant NCA or the EMA regarding the DHPC prior to dissemination.
- Preparation of the draft DHPC: The MAH prepares a draft DHPC using a template provided by the EMA. Where there are several MAHs of the same active substance for which a DHPC is to be issued, usually a single consistent message should be delivered. The template reflects the EMA guideline. It contains extensive details for the type of content, style and layout.
- Preparation of the communication plan: The MAH prepares a communication plan including the intended recipients and the timetable for disseminating the DHPC. Involvement of healthcare professionals organisations or scientific societies is advised to better target audiences and improve future dissemination.
- Submission to regulatory authorities: The MAH submits the draft DHPC and the communication plan (including the intended recipients and the timetable for disseminating the DHPC) to regulatory authorities according to the route of authorisation previously taken for the medicinal product. The regulatory authority should coordinate the review process by its scientific committees.
- Evaluation and authorisation: The EMA will coordinate the review of DHPCs within its scientific committees/groups as appropriate. Once the content of a DHPC and communication plan from the MAH are agreed by national competent authorities or the EMA, they should exchange the final DHPC and communication plan using the Early Notification System (ENS)*, and the EMA should coordinate any subsequent safety announcement.
- Dissemination: Typically, it is the responsibility of the MAHs to disseminate the DHPC. However, a competent authority may disseminate a DHPC in any situation where it considers that it is necessary for the continued safe and effective use of a medicinal product. The DHPCs are always disseminated in national languages. When medicinal products have been authorised at the EU level or consecutively in different member states, the DHPC proposal is prepared in English. Once the text of the DHPC is agreed, the MAH will prepare translations in the official national languages. The MAH is responsible for giving feedback about the dissemination of the DHPCs. The competent authorities need to be informed on the efficiency of the dissemination and on any difficulties met in the process
- Publication: The final step is the publication of DHPCs. EMA and NCAs may also decide to publish the final DHPC and disseminate it to relevant healthcare professionals’ organisations.
* The purpose of the ENS is to notify the European Union (EU) regulatory network (European Medicines Agency [EMA], National Competent Authorities [NCAs] and the European Commission [EC]) and international partners of emerging safety issues for which regulatory action and communication are envisaged. Notification is given every month in advance of the Committee for Medicinal Products for Human Use (CHMP) meeting. Before the end of the CHMP meeting, the completed communication material is disseminated within the EU regulatory network and to international partners prior to publication on the EMA website.
5. DHPC detailed processing
- The medicinal product has been authorised at the EU level (centrally authorised product or ’CAP’) or the product is subject to an ‘EU referral procedure for safety reasons’: The EMA coordinates the review of the DHPC within its scientific committees/groups and with NCAs.
- The medicinal product has been authorised via the ‘mutual recognition’ or ‘decentralised’ procedures: The ‘reference member state’ coordinates the process with the MAH, while the ‘concerned member states’ are kept informed of any proposed actions.
- For strictly nationally authorised medicinal products (‘NAP’): The MAH should submit the draft DHPC and any communication plan to the competent authorities of the member states where the product(s) is/are authorised.
A flow chart describing the entire processing of DHPCs is provided in Figure 1.
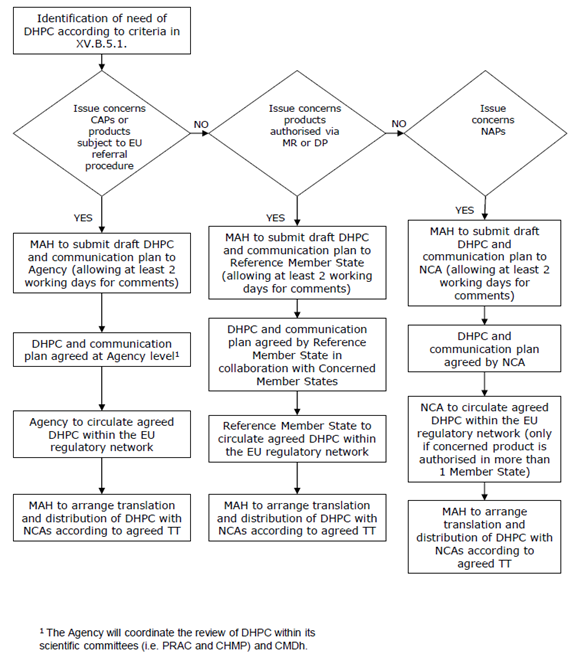
Figure 1: Flow chart for the processing of Direct Healthcare Professional Communications (DHPCs) in the EU (taken from the EMA guidelines document Guideline on good pharmacovigilance practices (GVP) - Module XV – Safety communication (Rev 1) (europa.eu))
TT = Time Table, NAP = Nationally Authorised Product, MR = Mutual Recognition Procedure,
DP = Decentralised
Procedure.
5.1. A national implementation of the EMA guidance: the Dutch example
The national authority in the Netherlands responsible for the processing of DHPCs is the College ter Beoordeling van Geneesmiddelen - Medicines Evaluation Board (CBG-MEB). Under a dedicated section on their website [3], the CBG-MEB gives access to the DHPCs that have been issued in the Netherlands since 1998. Several sections refer to what is written in the EMA guideline, the possible reasons for sending a DHPC and the procedure for DHPCs according to the three different routes of MA. The procedures that the MAH should follow when getting in touch with the CBG-MEB are also carefully described; in particular for a DHPC, when it is exclusively a national initiative. The website also provides details about specificities of the Dutch system. For example, the use of an ‘orange envelope’ – rather than a standard white envelope – is discussed for situations that require urgent action to be taken towards the patient. Routine methods of communication should not be used, according to the CBG-MEB, but special wording should be used to focus extra attention on the letter, such as ‘important restriction of indication’ or ‘very important warning that must be implemented immediately’.
6. Use of DHPCs
An article from 2014 explores the DHPC system in Europe and explores consistency in safety communications across multiple European regulatory agencies [4]. Of the 185 novel medicines authorised (via CP) between January 1st, 2001 and December 31st 2010, 53 (26%) were the subject of at least one DHPC safety communication. A total of 95 DHPCs were issued by the EMA between January 1st, 2001 and April 30th, 2013, including five withdrawals. The authors come to the conclusion that among the 4 European member countries with national regulators that make DHPCs publicly available since at least 2001, there were substantial inconsistencies in safety communications for novel medicines. The impact of those inconsistencies in terms of public health remains to be determined.
7. Conclusion
Disseminating safety information efficiently is one of the major topics in the Good Pharmacovigilance Practices in the European Union. The DHPC system is one of the most important tools to protect public health. Appropriate coordination and full cooperation between the EMA, the national regulatory authorities and the MAHs is essential to efficiently inform healthcare professionals and patients.