3. Public hearings
| Site: | EUPATI Open Classroom |
| Course: | Pharmacovigilance - Risk management |
| Book: | 3. Public hearings |
| Printed by: | Guest user |
| Date: | Friday, 20 June 2025, 5:45 PM |
Section Overview
- 1. Public hearings
- 1.1. Overall aim – TRUST
- 1.2. Definition of public hearing
- 1.3. Purpose of a public hearing in the context of safety referral procedures
- 1.4. When to hold a public hearing?
- 1.5. Who can attend?
- 1.6. Decision to hold a public hearing
- 1.7. Organisation of a public hearing: Before the hearing
- 1.8. Impact of a public hearing on PRAC opinion
1. Public hearings
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
Public hearings are a tool allowing the EMA to engage with European Union (EU) citizens in the supervision of medicines and listen to their views and experiences.
The EU's pharmacovigilance legislation [1] enables the PRAC to hold public hearings during certain safety reviews of medicines. They support the committee's decision-making by providing perspectives, knowledge and insights into the way medicines are used.
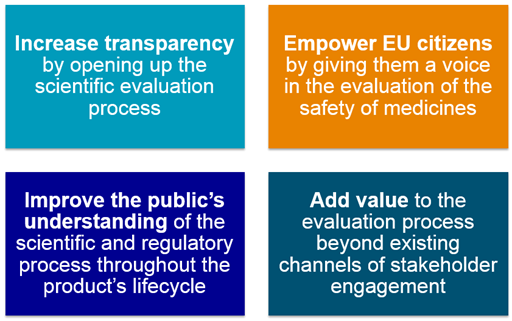
1.1. Overall aim – TRUST
1.2. Definition of public hearing
A public hearing is:
- A forum to which the public is invited to express its views and concerns on a pre-defined set of questions on issues related to the safety of a particular medicinal product, a medicinal substance or a therapeutic class, whilst also considering the therapeutic effects of these products.
- A channel for the PRAC to take the public’s views and concerns into account, particularly where options for regulatory actions and risk management activities will need to be considered in a wider public health context.
This input is of particular value once the PRAC has assessed all available data and is considering regulatory options to manage or minimize risks with a medicine in a wider public health context.
1.3. Purpose of a public hearing in the context of safety referral procedures
- To seek public opinion, suggestions and recommendations on the acceptability of the risks associated with the medicine or class of medicines concerned. This would be particularly in relation to its therapeutic effects and therapeutic alternatives available, as well as on the feasibility and acceptability of risk management and minimisation activities.
- To inform the debate of the PRAC, which continues to have the sole responsibility for giving its scientific recommendation on the safety of the medicine concerned.
1.4. When to hold a public hearing?
The hearing can be held at the beginning of a review on a safety concern of a medicine or later when the evidence is being evaluated.
Details of the discussion on holding a public hearing will be included in the PRAC’s minutes, which are published on the EMA website after each full meeting.
1.5. Who can attend?
Public hearings are open to all members of the public, including patients and healthcare professionals. The questions asked by the PRAC to be addressed during the hearing determine the target audience.
The marketing authorisation holder(s) has/have the opportunity to present its/their view(s).
Media organisations who wish to cover the public hearing may attend as observers.
PRAC members and EMA staff will also attend.
Modalities:
Participants will be asked to register in advance.
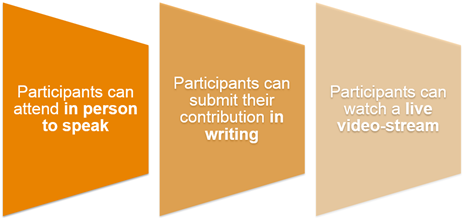
Members of the public can participate actively, as speakers, or they can opt to participate as observers.
Speakers can make an intervention in person or via teleconferencing. Supporting documentation presented by the speakers during the intervention will be published on the EMA website following the public hearing.
Where space permits, requests to observe the public hearing in person without making an intervention will be accommodated.
The proceedings of the public hearing can also be observed via video broadcast on the EMA website.
1.6. Decision to hold a public hearing
The PRAC decides when to hold public hearings on a case-by-case basis, depending on the urgency of the matter and on other justified grounds, particularly with regard to the extent and seriousness of the safety issue.
Proposal for a public hearing
- Referral procedures in accordance with Art. 20 of Regulation (EC) 726/2004, Art. 31 or Art. 107i of Directive 2001/83/EC
- Proposal to hold a public hearing can be submitted by any PRAC member
- Content of proposal
- Purpose of the public hearing (what is intended to be achieved?)
- Specific questions on which public opinion should be sought
- Any additional information as appropriate
Evaluating the need
At the start of each referral the PRAC will consider the need to hold a meeting and the following points:
- Feasibility to hold a public hearing in light of the urgency of the matter.
- Nature and extent of the safety concern.
- Therapeutic effect of the medicine/class of medicines and availability of therapeutic alternatives.
- Potential impact of possible regulatory actions on therapeutic practice and availability of treatments.
- Level of public interest.
Agreement to hold a public hearing will be reached by consensus or, if not, by majority vote.
1.7. Organisation of a public hearing: Before the hearing
The Announcement of the public hearing published on the EMA website, together with:
- A summary of the safety concern
- A list of specific questions
- Information on date, time and location of the public hearing
- Registration information
- Information on how to submit written contributions
- Contact email address and phone number
- Information about live-broadcast/web stream
Submitting a request to speak
Participation requests should be sent in writing to the EMA using a dedicated
form and should include the following information:
- Capacity (e.g. patient, carer, doctor)
- Affiliation: Name of the organisation/group being represented (if applicable)
- Contact information
- Outline of the planned presentation/ specifically how it addresses the questions on which the PRAC is seeking public opinion
- Declaration of interest pertaining to the medicine(s) to be discussed at the public hearing
Review of requests to speak:
- All requests will be reviewed
- Efforts will be made to accommodate all speaking requests
- Speaking requests may be declined only if clearly unrelated to the subject matter of the public hearing
- Priority will be given to civil society representatives, such as patients, consumers, healthcare professionals and academic research groups or organisations, particularly those relevant to the therapeutic area
- Confirmation of attendance as a speaker at least 10 days in advance of the public hearing
1.8. Impact of a public hearing on PRAC opinion
- The information gathered in the public hearing informs the debate within the PRAC
- The contributions made by the public during a public hearing will be considered by the PRAC
- The assessment report will reference how the outcome of the public hearing will be addressed in the decision-making at the level of the PRAC
For more information please refer to the following texts:
Rules of
procedure on the organisation and conduct of public hearings at the
Pharmacovigilance Risk Assessment Committee (PRAC)
https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/rules-procedure-organisation-conduct-public-hearings-pharmacovigilance-risk-assessment-committee_en.pdf
Public
hearings Guidance for participants
https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/public-hearing-guidance-participants_en.pdf