3. Studies in Pharmacoepidemiology and their classification
| Site: | EUPATI Open Classroom |
| Course: | Epidemiology and Pharmacoepidemiology |
| Book: | 3. Studies in Pharmacoepidemiology and their classification |
| Printed by: | Guest user |
| Date: | Wednesday, 2 July 2025, 4:03 AM |
1. Studies in Pharmacoepidemiology and their classification
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
Pharmacoepidemiologic studies offer assessments of potential short and long-term benefits and risks of a medicine and other outcomes within a population with a wide range of health status and demographic characteristics and with a longer follow-up period than in clinical trials, that measure efficacy and safety. Studies clarify clinical and demographic characteristics of diseases and conditions; they identify who is at risk and provide clues to the causes of disease. Studies include the analysis of prescribing medication and its determinant factors, give guidance to preventive measures and interventions, describe and analyse the economics of medicine use and underpin scientific advice to decision-makers.
The abundance of digital data has facilitated pharmacoepidemiology and observational research on the effectiveness of medication use under real-world conditions (providing so-called Real World data, RWD). Advantages of pharmacoepidemiologic research are the availability of data from larger groups of patients, and coverage of under-researched subpopulations, allowing more diverse populations to be looked at compared to clinical trials. Drawbacks of observational pharmacoepidemiologic studies come from the non-randomised nature of group selection, leading to various kinds of possible interference by confounding factors (variables). Potential methods for managing this drawback include for instance active comparison groups, within individual designs. Many of the more rigorous pharmacoepidemiology studies have been strengthened through multiple analytical approaches to improve confidence in inferred causal relationships.
In the following a few principles and types of epidemiological studies as employed in pharmacoepidemiology are covered.
1.1. Descriptive or analytic
Epidemiologic studies often are divided into two types, descriptive and analytic, and each of these uses specific kinds of studies.
Descriptive studies examine patterns of disease occurrence, with a focus on person, place, and time. These studies use relatively accessible data to estimate caseloads, to determine the amount of public health resources needed, or to identify high risk groups. Descriptive studies are useful if very little is known about a new disease or condition. They can be used to generate hypotheses on risk factors and causes of disease that need to be confirmed or ruled out by analytic studies. For example, early descriptive studies found that the majority of newly diagnosed AIDS cases in the USA were among young men having sex with men, which led to the hypothesis that certain types of sexual behaviour might cause AIDS. The most basic types are: a case report, a case series, and an incidence study. These types of study involve no comparison group. They are merely descriptive.
Analytic studies aim to identify and evaluate determinants, causes or risk factors of diseases or health-related events. In contrast to descriptive studies, which generate hypotheses, analytic studies are used to test hypotheses. They are often employed to analyse the distribution of exposures and diseases and to look for and measure associations. These types of studies typically require more resources and expertise and can be rather complex. A key feature of analytic studies is that they involve comparison groups.
1.2. Experimental (interventional) or non-experimental (observational)
Experimental (interventional) and non-experimental (observational) studies are two different approaches to detect and assess an association and establish possible causation, for instance between disease and exposure to an assumed risk factor or between disease and exposure to a therapeutic intervention, such as a medicine. The terms experimental or interventional and non-experimental or observational are often used interchangeably and across several types of research.
The key difference between experimental and observational studies lies in the extent to which the environment is controlled and determined (‘manipulated’) by the researcher.
Experimental studies have the distinguishing characteristic that the intervention being tested (e.g., preventative or therapeutic measure) is allocated by the investigator to a group (sample) of study participants drawn from a population (individuals, households, communities). Participants are followed prospectively to compare the intervention vs. the control (standard treatment, no treatment or placebo) and detect the effects of the intervention (exposure). The main interventional study design is the randomised controlled trial (RCT) (randomised, meaning the participants are grouped by chance).
Observational studies take place in a real-world setting, and it is not possible for the researcher to control all possible variables. Observational studies therefore lack the ‘manipulation’ of an independent variable, control of extraneous variables through random assignment of study participants, or both. Researchers observe the effect of risk factors, or health interventions, without trying to change who is or isn’t exposed. Observational studies come in four main types: Cohort, case-control, cross-sectional, and ecologic (see below). Causality is difficult to demonstrate with these designs; that is, in general they allow to draw conclusions regarding association. To note, there are circumstances in which experimental designs are not appropriate (typically for ethical reasons) or impossible. Moreover, long-term studies that span several years or decades are difficult to set up as experimental studies. Observational studies tend to be more flexible and allow for a greater range of topics to be studied.
1.3. Overview of study types
The following figure shows a schematic of study types as used in pharmacoepidemiology.
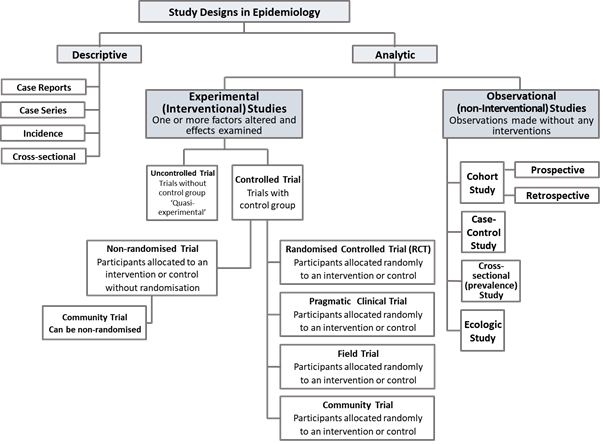
Figure 1: Schematic of study types in Pharmacoepidemiology. To note: Depending on the specific design Cross-sectional studies can be descriptive or analytical as well as ecologic studies; community trials can be randomised or non-randomise.
