4. International Council on Harmonisation (ICH)
| Site: | EUPATI Open Classroom |
| Course: | Introduction to Regulatory Affairs |
| Book: | 4. International Council on Harmonisation (ICH) |
| Printed by: | Guest user |
| Date: | Wednesday, 25 June 2025, 11:40 AM |
1. International Council on Harmonisation (ICH)
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
The European Commission recognised the economic burden of different national and regional requirements to MA applications, seen as an issue also by the regulatory authorities in the USA (Food and Drug Administration or FDA) and in Japan (Ministry of Health, Labour and Welfare, MHLW). In the early 1990s, the European Commission took the initiative to establish the International Conference on Harmonisation ICH http://www.ich.org/home.html in order to reduce the duplication of clinical trials and create a more streamlined regulatory assessment process for new applications. The first ICH conference took place in Brussels in 1991. In addition to regulatory authorities, industry organisations from the three regions participated in the conference (two more regions have been added since then, Canada and Switzerland). Finally, several observers joined the group.
In 2015 there was a need to expand the ICH scope from three, and later, five regions transforming it into a formalised global association. At the end of 2015 the ICH has changed to become the International Council for Harmonisation. The ICH Association is registered as a legal entity under Swiss Law. Their rules stipulate that the association establishes an ICH Assembly (https://www.ich.org/page/members). It retains the original acronym, ICH. The Assembly is open for more members than the original ICH.
A formal structure for collaboration is agreed. The primary goal for the ICH remains to elaborate (develop) and agree upon harmonised guidelines for medicines development and regulation. Such guidelines can considerably reduce the extra work imposed by different documentation requirements in the participating regions. A Management Committee (formerly a Steering Committee) controls the work.
The Management Committee consists of the following:
- Regulatory Authorities
- Europe - EU Commission (and the European Medicines Agency or EMA),
- USA – FDA,
- Japan – MHLW,
- Canada, Health Canada,
- Switzerland, Swissmedic,
- eight further regional or national regulatory authorities elected by the ICH Assembly.
- Industry organisations
- Europe - European Federation of Pharmaceutical Industries and Associations (EFPIA),
- USA - Pharmaceutical Research and Manufacturers of America (PhRMA),
- Japan - Japan Pharmaceutical Manufacturers Association (JPMA),
- four further regional or national industry organisations elected by the assembly.
- Observers
- World Health Organisation (WHO).
- International Federation of Pharmaceutical ‘Manufacturers & Associations (IFPMA).
- Support
- ICH Secretariat.
One unique aspect of the ICH organisation is that industry participates formally in the Management Committee as well as in the work in general. This arrangement had never before existed nationally or regionally. In any case, the authorities still have the final say in any decisions.
There are four working groups doing the concrete work of elaborating ICH guidelines. The four groups each cover a specific area:
These groups work in areas covering several aspects of medicines development. As such, ICH has developed four sets of guidelines provided for the specific topics quality, safety, efficacy, and multidisciplinary (e.g. MedDRA, the Medical Dictionary for Regulatory Activities http://www.meddra.org/ which looks to provide standardised medical terminology, or the Common Technical Document -CTD- which are implemented by the regulatory authorities of its membership). For details, see section 4.2.
1.1. Elaboration of ICH guidelines
The ICH Expert Working Groups (EWG) follow a formalised structure when elaborating guidelines.
- Before starting a project to develop or elaborate a new guideline, the EWG drafts a concept paper.
- The Management Committee must endorse the project.
- The EWG then appoints a rapporteur. This rapporteur may be an industry delegate or a regulator.
- The entire membership of the EWG will need to agree on the draft guideline - called the ‘Technical Document’ [Step 1].
- Next, the regulators will formally adopt the draft guideline and publish it for external consultation in the ICH regions [Step 2].
- In Step 3, the EWG will discuss the comments received during the consultation period. Industry delegates still participate in the discussions. However, if the initial rapporteur was from industry If the initial rapporteur was from regulatory, no changes are necessary.
- In the adoption phase only the regulatory members will sign off as the guideline is formally a regulatory document. [Step 4].
- Delegates from observers may participate in EWG meetings and discussions but without any voting rights.
- Implementation of new ICH guidelines will follow regional procedures [Step 5]. In the EU, a new ICH guideline is tabled for the Committee for Medicinal Products for Human Use (CHMP) who will formally adopt it. The guideline is published similarly to EU guidelines. The new guideline is labelled as a CHMP/ICH guideline.
Many guidelines have been developed via ICH collaboration: (accessed on ICH website January 2021)
1.2. ICH Common Technical Document (CTD)
Most of the ICH guidelines contain information for industry on what documentation to provide in a MA application. This helps industry to reduce duplication of work. For example, without harmonised guidelines relating to ‘efficacy’, extra burden would be on companies. They might need to perform extra clinical trials to comply with the different requirements in different regions.
The format of the dossier submitted for a MA is also important. It is helpful for everybody involved if dossiers for different regions can be structured in a similar way. In 2000, the ICH published a very important guideline in this area, the ‘Common Technical Document’, known as the CTD.
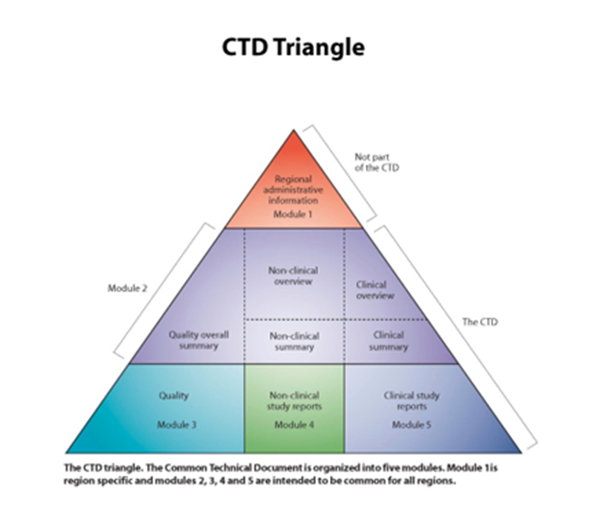
Figure 1: The CTD Triangle showing the content of the CTD.
CTD Triangle The CTD Triangle. The Common Technical Document is organized into five modules. Module 1 is region specific and modules 2,3,4 and 5 are intended to be common for all regions. Source: www.ich.org
The CTD Triangle. The Common Technical Document is organized into five modules. Module 1 is region specific and modules 2,3,4 and 5 are intended to be common for all regions. Source: www.ich.org
The CTD describes in detail how a dossier for a MA application shall be collected and structured. A description of the content appears in Figure 1 above. The file for an MA application consists of five modules. The triangle illustrates that the broader the section, the larger the amount of documentation required.
-
Modules 3, 4 and 5 (widest row of the triangle) - contain the full and detailed documentation covering quality, safety and efficacy. For a new innovative medicine, this may well be 200,000 A4 pages or more.
-
Module 2 (middle row of the triangle) - as Modules 3, 4 and 5 are so lengthy - summaries have to be included to improve the readability of the dossier.
These summaries form Module 2:
- For quality, one summary document is sufficient – an overall summary.
- A two-level approach is needed for non-clinical and clinical - both having a summary and an overview. The summary documents mentioned may be up to a few hundred pages. The overview documents are condensed versions of typically 30 pages.
-
Module 1 (narrowest row of the triangle) - this is not harmonised between regions. Each region has to elaborate (develop) its own Module 1. The application form and proposed labelling are amongst the elements of this module.
The structure of the CTD makes it more convenient for the experts assessing the dossier. They will normally start with the overviews and summaries to get a broad picture of the documentation on the new medicine. After that, they can dig deeper into the detailed documentation whenever needed to fully understand and evaluate the dossier.
1.3. Electronic versions of MA applications – e-submissions
A paper based MA application dossier is a logistical challenge. More than 200,000 pages per dossier takes up a lot of physical space in the assessment phase as well as an enormous amount of space in both local and remote archives. Recently a case has been reported where the full dossier comprised 18,000 000 (18 MILLION!) A4 pages. When the CTD was finalised, work progressed in developing a standard for an electronic version, an eCTD. This standard is a detailed guidance on how to combine all the individual electronic documents into a folder and file structure.

Today an application to the EMA for the centralised procedure can only be submitted as an eCTD. Paper applications are no longer accepted. The same is true for some member states (MS) when it comes to other application procedures. Documentation in paper is still accepted in a number of countries, particularly for national applications.
The most convenient way of submitting and receiving electronic applications is via an e-portal. The EMA has a portal for uploading applications under the centralised procedure. Many MS have developed similar portals. For national and mutual-recognition (MRP) or decentralised (DCP) applications, applicants would need to upload their e-submissions via a large number of different national portals. To avoid this, the National Competent Authorities (NCAs) of a large number of member states have developed a common portal. This is called the Common European Submission Platform (CESP).
Figure 2: Diagram highlighting the member states that participate in the Common European Submission Platform (CESP).
A majority of regulatory agencies, for both human and veterinary medicines, participate in the CESP project. The project is controlled via agreements in the HMA (Heads of Medicines Agencies).
Most of the other ICH partners outside Europe have only one medicines agency. This means that the logistic problems in creating portals for industry to upload e-submissions are far less, as each country requires only one portal to upload an eCTD.