Trial Participants: Informed Consent, GCP, Patient Involvement
4. Patients Can Get Information From Their Healthcare Professionals
4.1. The Next Steps
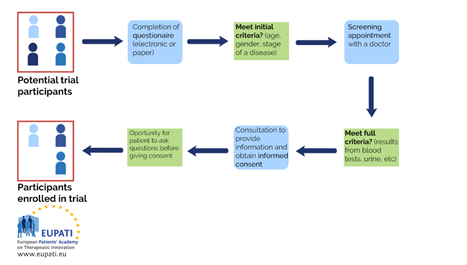
When a patient has decided that they would like to enrol in a trial, there are several essential steps that need to be followed before they can ‘sign up’:
- Enrolment - is the process of signing up or recruiting participants into a clinical trial.
- Eligibility - the number of participants enrolled in a study varies greatly depending on the phase of the trial and the trial design. It must be found out whether a participant will meet the requirements outlined in the protocol
(inclusion/exclusion criteria) to join the trial.
- Informed Consent - the participant can then sign the written informed consent after going through the process with a suitably qualified person, usually a member of the research team.
Those who meet the initial requirements are then invited for further screening, usually with a doctor or other healthcare professional directly involved in the trial.
Once the screening determines that the patient meets the inclusion criteria, the patient has a consultation where more and detailed information about the trial is provided, questions can be asked and an informed consent is signed (or refused).