Principles of New Trial Designs and their Practical Implications
3. Possible Approaches in Adaptive Design
3.3. Example 3: Seamless Phase II/Phase III Design
Seamless Phase II/Phase III design is often used in the case of rare diseases; it is also called a ‘combination test’. In the example below, patients are randomised between three treatment arms in the first stage of the design (Phase IIb). The first treatment arm is the control arm, where patients receive the standard of care therapy. Patients on the second and third treatment arms receive different treatments, Treatment 1 or Treatment 2.
At the end of the first stage (Phase IIb), Treatment 1 and Treatment 2 are compared based on best progression-free survival (PFS). The least effective treatment arm is dropped. The other treatment arm is then continued in the second stage (Phase III). In this stage, an efficacy comparison is performed against the standard of care treatment.
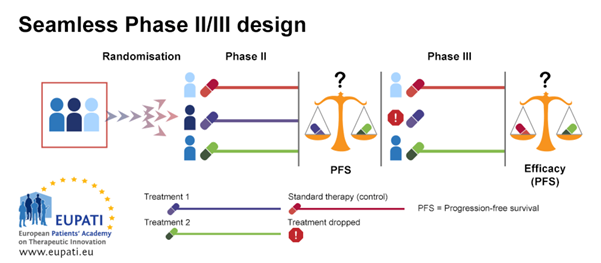
Seamless
Phase II/III design
The seamless Phase II/III design allows Phase II and Phase III to be performed
in the context of one trial.
Advantages in Seamless Phase II/Phase III design
- Helps to mitigate bias
Both steps are conducted independently and the results of both steps are combined in the end in an overall test result.
- Shortens time and participant exposure
Phase II and Phase III are performed within the context of one trial.
-
Relatively flexible
The way that the treatment arm for final comparison is chosen in the Phase II part and merged with the Phase III part is relatively flexible.
-
Efficient use of resources
Participants from Phase II and Phase III both contribute data to the final results.
Disadvantages
- Complicated statistical analyses
This design requires statistical aspects that are not so straightforward.
- Recruitment gaps
There is a gap in the recruitment between the two phases while waiting for enough data to be gathered in order to perform the interim analysis that decides whether to continue or not.
- Logistic challenges
This design is logistically challenging – it requires a quick flow of data so that the number of events in the analysis can be followed up on.
- Difficulties arising from long-term endpoints
This design requires information on PFS to be available relatively quickly. This becomes more difficult when the endpoints are long-term.
- Risk of lost information
Combining two arms risks the loss of information.