Bioavailability and Bioequivalence
| Site: | EUPATI Open Classroom |
| Course: | Development of Medicines Substance and Final Medicines Product |
| Book: | Bioavailability and Bioequivalence |
| Printed by: | Guest user |
| Date: | Wednesday, 2 July 2025, 3:42 AM |
1. Bioavailability
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
Medicines contain an active substance, also
referred to as Active Pharmaceutical Ingredient (API) and they are used in order to cure, treat or
prevent illness in human beings or in animals. But they can also be used for
other purposes, such as diagnosis of certain diseases. In all these use cases, the
API must be able to enter the body. But in order to have a therapeutic effect, the
API is also needed in the correct dose at the specific site in the body where
it has to work. This specific site is referred to as the target site. In
addition, the API needs to reach the target within a certain time and to stay
there for a defined period.
The rate and degree to which the API is
available at the target site is known as bioavailability. This comes closest to
defining a “true” or “ideal” bioavailability for a medicine. It is mirrored in
the definition by a regulatory authority, the United States FDA. They define
bioavailability as "the rate and extent to which the active drug
ingredient or therapeutic moiety is absorbed from a drug product and becomes
available at the site of drug action", whereas the European EMA simply
defines it as “The extent to which an active ingredient is absorbed from a
medicine and becomes available in the body”. The latter, while less precise, is
however closer to what is measured in reality.
Determining bioavailability
A few points upfront:
- Bioavailabilty, the fraction of the administered
dose which reaches the systemic circulation unchanged, is denoted by the letter f and, if expressed as percent (which is
usually the case), by the letter F.
- Calculation is based on the assumption that there is
a direct relationship between the concentration of drug in blood or plasma
and the concentration of drug at the site of action. However, consider the possibility that
circulating drug levels may misrepresent its availability to its target.
(Example: as the only biologically active form of the medicine phenytoin
is the free drug, the circulating protein-bound fraction (up to 90%) is
not available to its site of action in the brain, even though it might be
well-absorbed).
- When a
medicine is given orally, only part of the administered dose appears in
the plasma.
(Example: if 100 mg of a medicine are administered orally and 70 mg of this medicine are absorbed unchanged, the bioavailability is 0.7 or seventy percent). - Various factors may affect bioavailability, such as Pharmaceutical factors, Patient related factors, Route of administration, metabolism
The most common type of human bioavailability studies are Plasma Level - Time Studies. Following the administration of a single dose of a medication, blood samples are drawn at specific time intervals and analyzed for drug content
1.1. Intravenous bioavailability
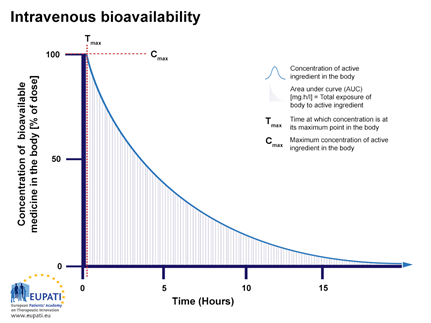
When giving an intravenous injection (IV) directly into the bloodstream, the bioavailability is by definition 100%. Figure 1 shows the bioavailability profile of medicines given IV. This is shown as concentration over time in hours.
Figure 1: The percentage of active substance in the body or bioavailability, after direct injection into the blood stream and observed over a period of 15 hours. The area under the curve (AUC), is shaded. Tmax is the time when the highest concentration of the medicine is found in the blood, Cmax is the maximum concentration.
After injection, an API reaches the target site after a complex journey in the bloodstream and body.Immediately after injection, the bioavailability will be 100% (seen on the y-axis in Figure 1 as Cmax and on the x-axis at Tmax). So, if 75 milligram (mg) of active substance is injected into the bloodstream, then 100% corresponds to 75 mg active substance. The concentration of the API in the body will then decrease over time (Figure 1) because, a fraction of it will be metabolised or excreted.
1.2. Intravenous vs. other route
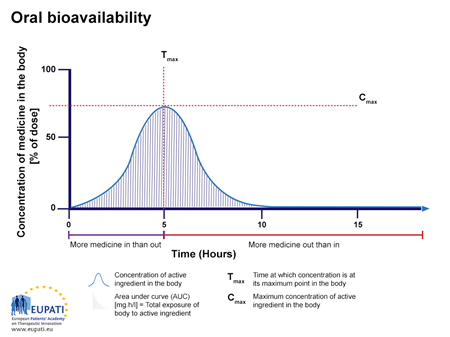
If the same API, as shown in Figure 1, is given via another route, such as a tablet given orally (by mouth), the bioavailability is lower than 100% and the time course is different (Figure 2).Figure 2: The concentration of medicine (in % of dose administered) in the blood for a tablet taken orally, and observed over the period of 15 hours. The area under the curve (AUC) is shaded. Tmax is the time when the highest concentration of the medicine is found in the blood, Cmax the maximum concentration.
The lower bioavailability of the oral route compared to injection is explained in the following example from Figure 3. After a tablet or capsule is swallowed, it reaches the stomach within a minute or two. In the stomach, it is then dissolved and some of the active substance is absorbed into the bloodstream.
An image which compares the absorption of medicine from an intravenous injection with that of a medicine from a capsule taken orally.
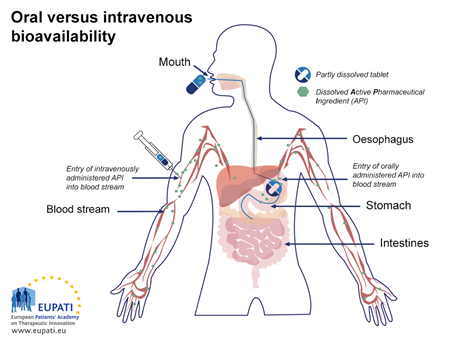
Figure 3: Schematic illustration of absorption from a capsule taken orally (by mouth) versus injection directly into the bloodstream (IV). After reaching the stomach, the capsule is further transported to the small intestine, where further absorption occurs.
After some time in the stomach, the components are transported to the small intestine. Here they will be fully absorbed. Absorption from the gastrointestinal tract can vary greatly. A poor absorption or a lack of absorption from the stomach and the intestines can lower the bioavailability. When the API is absorbed, it first reaches the hepatic portal vein, and is then transported to the liver. This is the first time the active substance is metabolised in the liver, which is called the ‘first pass metabolism’. Some APIs are metabolised more than others during this first pass metabolism. The non-metabolised part of the active substance, normally less than 100%, will reach systematic circulation via the hepatic vein. The amount that actually reaches systemic circulation is referred to as the ‘absolute bioavailability’.
1.3. Example 1
Today, a certain type of penicillin can only be given as IV injection. However, such IV treatment must be administered at the hospital. A company has now developed a tablet containing penicillin which will make it easier and possible to treat patients in their home. The company would like to put the tablet on the market for patients. Before doing so, the authorities request the company to compare the effect of the tablet with that after injection which is commonly used. They want to test bioavailability of the medicine in the new form.1.4. Absolute Bioavailability
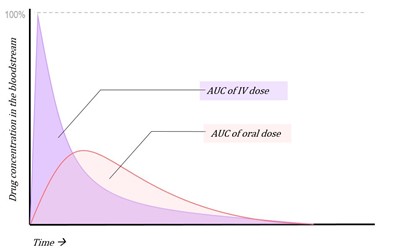
Figure 4: Concentration vs. time graph of AUC of IV and oral administration of a medicine
The bioavailability of the intravenous dose of any drug is by definition 100%. Hence, the bioavailability of all other formulations and routes of administration can be compared to this reference value as an absolute standard, and from this the equation for absolute bioavailability can be derived:
The absolute bioavailability then is the dose-corrected area under the curve (AUC) non-intravenous divided by AUC intravenous. The equation for calculating the absolute bioavailability (denoted by the letter f or, if expressed in percent, by F), of a drug administered orally (tablet) is given below:
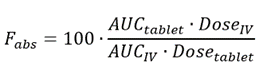
1.5. Example 2
Now let’s try to use the equation to calculate the bioavailability of a penicillin given as IV versus given as a tablet. Let’s say that the AUC of 25 mg penicillin after administered as IV is 100 mg.h/L and that the AUC is 60 mg.h/L after giving
a tablet containing 100 mg penicillin. What then is the absolute bioavailability of the penicillin?
The values given in Example 2 are inserted into the equation:
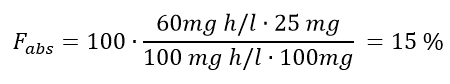
Thus, the absolute bioavailability of such a tablet is 15%.
As seen in Figures 1 and 2, the concentration – time profiles differ for the tablet and IV injection (the two formulations). And it is even more complicated than this. The profile obtained between them would likely differ to some extent if you made a similar profile for the two formulations using ’Matt’ and ‘Per’ as test persons. This is because bioavailability is affected by a number of parameters within individuals.
1.6. Example 3 - relative bioavailability
Brian suffers from breathing problems when he plays football. The doctor would like Brian to be able to treat future asthma attacks himself. He therefore considers giving Brian either tablets or an asthma spray, both containing the same active substance. But which parameters do you think will affect the relative bioavailability?What you have to consider is that Brian has trouble breathing, so he needs the API to reach the target site in the body as fast as possible.
The target site for asthma medication is the lungs. Let’s forget everything about side effects for a minute and whether Brian prefers to receive an injection or taking a tablet. We will instead focus only on bioavailability. We have already talked about absolute bioavailability when we compared the AUC for an IV injection versus a tablet. Now we want to compare two formulations containing the same API, but administered via different routes.
Let’s calculate the relative bioavailability of an inhalator versus a tablet as was suggested to Brian in Example 3. The AUC for the inhalation is 20 mg h/L while the AUC for the tablet is 60 mg h/L. The dose given for inhalation is 1 mg whereas the tablet contains 5 mg. What is the relative bioavailability of the inhalation versus the tablet? And what would you recommend Brian to choose?
To calculate the relative bioavailability the following equation (relative bioavailability equation) must be used.
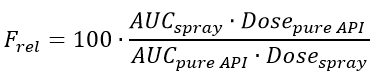
Then, the values given in Example 3 are inserted to the equation.
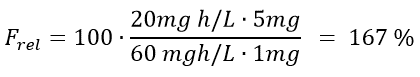
The result is that the absolute bioavailability of the inhalation compared to the tablet is 167%. You can therefore conclude that for this example, more API from the inhalation spray reaches the target site compared to the tablet. This is most likely due to a high degree of first pass metabolism of the substance after it is given orally. Therefore, Brian should be advised to use the inhalator. Another advantage of using the inhalator is that it will administer the active substance locally because the lungs are the target site. A tablet, on the other hand, needs to be dissolved in the gastro intestinal tract before uptake can take place. This would increase the risk of side effects because the API will be present in the systemic circulation. When an API is administered locally, the amount of it that reaches systemic circulation is minimized and so is the risk of systemic side effects.