Gene Therapy
| Site: | EUPATI Open Classroom |
| Course: | Types of Medicines and Their Mode of Action and Use |
| Book: | Gene Therapy |
| Printed by: | Guest user |
| Date: | Wednesday, 25 June 2025, 9:01 PM |
Section Overview
1. General Features
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
Gene therapy is a highly experimental technique that has shown promise in some clinical trials in humans for diseases such as immunodeficiencies (where the immune system does not function properly), muscular dystrophy (progressive loss of skeletal muscle) and anaemia (decreases in the number or function of red blood cells).
The gene therapy approach is useful for diseases in which a single gene is affected, either by a single mutation or multiple mutations in the same gene. In diseases which involve multiple genes, this approach is much more complicated and is unlikely to work.
2. Mode of action
There are three main gene therapy strategies:- Adding a ‘corrected’ copy of the gene.
- Knocking down or inactivating a mutated gene that is not functioning normally.
- Repairing a faulty gene.
In the first strategy of introducing a corrected copy of the gene into the body, the DNA has to be inserted in suitable packaging, called a vector. The vector protects the DNA from degradation and ensures that the gene is delivered efficiently to the target cells. Viral vectors are commonly used for gene therapy because viruses are highly effective in delivering genetic material into human cells. While the diseased cells will still produce the faulty protein, the presence of the new gene will mean that the cell will start to produce normal proteins as well. Some functionality will be restored, which should have a therapeutic benefit.
Knocking out a gene refers to the prevention of damaged proteins being made. When a protein is made, a ribonucleic acid (RNA) copy of the gene is made and transported out of the nucleus into the part of the cell where the protein synthesis machinery is located. Protein-coding RNA can be inhibited and targeted for destruction by small RNA molecules with a complementary sequence, thus preventing the faulty proteins from being synthesised by the cell.
The final gene therapy strategy is to repair the mutation in the DNA. This approach is high-risk as it involves altering the original DNA blueprint of the cell. As of 2014, this type of genetic engineering has not yet been tested in humans but has been used in human cell lines in the laboratory. This technique involves cutting out damaged sections of DNA and replacing them with the correct sequence.
The gene therapy vector can be introduced directly into the body via injection or inhalation, or cells can be modified in the laboratory before insertion into the body. Once the gene therapy vector is inside the body it will deliver its cargo to cells and the DNA will become integrated into DNA in the nucleus in some cases. The cell will use this new DNA to start making corrected copies of the protein.
3. Production Process
- DNA Engineering - see subchapter 3.1
- Viral vectors - see subchapter 3.2
- Non-viral vectros - see subchapter 3.3
3.1. DNA Engineering
Recombinant DNA technology is used to engineer the corrected copy of the gene in the laboratory. This could be part of a gene, or the whole gene. This process involves sticking together different sequences of DNA to make a functional gene. Researchers use bacteria to produce large amounts of this engineered DNA because they are highly efficient at making DNA. Generally, smaller genes are easier to deliver to cells than large genes. The DNA of the corrected gene is inserted into the vector, which will protect the DNA during its journey into the body and to the affected cells (Figure 1.1). Gene therapy agents are produced on a very small scale as the process is extremely labour-intensive.
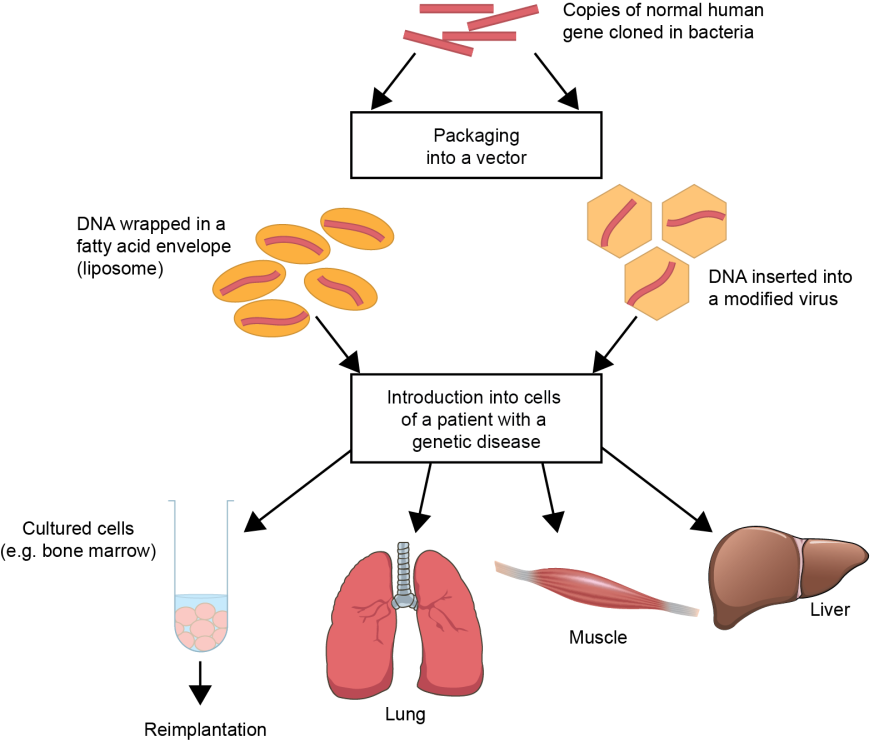
Figure 1.1. Overview of gene therapy . Normal DNA sequences are engineered in the laboratory and multiple copies are made by bacterial cells. The DNA is then inserted into a gene therapy vector, either viral or non-viral. Liposomes are an example of non-viral vectors and are small membranes made of fatty acids, which can easily encapsulate the DNA. The gene therapy vector is then introduced to cells, either, directly into the body via injection or inhalation, or cells are modified in the laboratory before implantation into the body.
3.2. Production process - Viral vectors
Viruses are commonly used as vectors. They have had specific modifications made to render them non-infective. Often they are designed to last a long time inside the affected cell, either by integrating into the cell’s DNA or staying inside the cell. This reduces the requirement for repeated treatments. The main safety concern when using these agents is the risk of insertional mutagenesis. This is when a piece of DNA enters the nucleus and causes disruption of the surrounding genes, such as those that normally suppress tumour growth. In several gene therapy trials, some patients have developed leukaemia as a result of insertional mutagenesis. In these cases, the gene therapy DNA inserted itself close to a gene, which can cause cancer if switched on.Additionally, the introduction of viral proteins into the body can activate the immune system too as if the body has been infected, which can make the patient receiving gene therapy ill. Owing to these safety concerns, and other issues surrounding genetic engineering in humans, the European Medicines Agency has published guidelines for the development and use of gene therapy vectors in humans.
3.3. Production process - Non-viral vectors
In light of the safety concerns relating to the use of viral vectors, non-viral delivery methods have been developed for DNA delivery, such as fatty acid envelopes (liposomes) and coating metals in DNA (the ‘gene gun’). Non-viral gene therapy vectors can be produced on a much larger scale than viral vectors.4. Mode of administration
5. Example - Gene therapy for X-linked severe combined immunodeficiency
Gene therapy has been used successfully to treat X-linked severe combined immunodeficiency (SCID-X1), an inherited disease in which the body produces very few immune cells. Therefore, patients are prone to multiple infections, which can be fatal. It is caused by a mutation in a gene located on the X chromosome, IL2RG, which is why it is called an X-linked disease.
Some of the first successful human gene therapy trials were conducted in patients with SCID-X1, which allowed participants to live relatively normal lives. In one trial, individuals were given gene therapy to introduce a functional version of the IL2RG gene into their bone marrow cells. Bone marrow cells were harvested from patients, then modified outside of the body and infused back into the patients. After 10 years, seven out of nine patients had functional immune systems. During the study, one patient died and four developed acute leukaemia, although all four recovered. An increased risk of leukaemia has been observed in other similar trials and as previously discussed, has been attributed to insertional mutagenesis caused by the use of viral gene therapy vectors.
6. Example - Gene therapy for cystic fibrosis
7. Example - Gene therapy for Duchenne muscular dystrophy
8. Further Reading
- European Society of Gene and Cell Therapy , 2014. What is gene therapy? Available at: What is gene and cell therapy? | EuroGCT (Accessed 3 March 2014).
- Adventures in Synthetic Biology produced by Drew Endy, Isadora Deese, and Chuck Wadey. 2005 (http://web.mit.edu/endy/www/scraps/comic/AiSB.vol1.pdf )
9. References
- European Medicines Agency . Multidisciplinary scientific guidelines on the use of gene therapy. Available at: Multidisciplinary: gene therapy | European Medicines Agency (europa.eu)