Medical Devices and Medicine-Device Combinations
| Site: | EUPATI Open Classroom |
| Course: | Types of Medicines and Their Mode of Action and Use |
| Book: | Medical Devices and Medicine-Device Combinations |
| Printed by: | Guest user |
| Date: | Wednesday, 25 June 2025, 2:52 PM |
1. General features
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
Medical devices are the most widely used medical products. More than 10 000 categories of devices exist in the world and so have a diverse range of features. Medical devices can be classified according to their uses into the following:- diagnosis, prevention, monitoring, treatment or alleviation of disease
- diagnosis, monitoring, treatment, alleviation of or compensation for an injury
- investigation, replacement, modification, or support of the body or of a physiological process
- supporting or sustaining life
- control of conception
- disinfection of medical devices
- providing information by means of examining samples taken from patients (for example, blood tests or biopsies )
In addition, certain products are classified as medical devices in some regions of the world but not in others, and include:
- disinfection substances
- aids for persons with disabilities (also called assistive products)
- devices incorporating animal and/or human tissues
- devices for in vitro fertilisation or assisted reproduction technologies.
2. Mode of action
How each medical device works will depend on what it is used for. For example, spectacles are a relatively simple device that alters the way that light enters the eyes. This corrects eyesight for the user. A more complex device, such as a blood glucose meter, works to monitor the levels of sugars in the blood. This is important in diabetes mellitus because these patients cannot regulate their blood sugars normally. If blood sugar levels increase or decrease too much then the effects can be harmful. Therefore, careful monitoring of blood sugars, along with the use of insulin to manage blood sugar levels, is important for patients with diabetes.3. Production process
Some of today’s medical devices, such as the tongue depressor, thermometer and stethoscope have been unchanged for centuries, while ‘bionic eyes’ for use in patients with retinitis pigmentosa, a severe eye disease, received regulatory approval for use in humans as recently as 2014. However, rather than discovery in a eureka moment during laboratory exploration, the creation of new medical devices results primarily from clinicians’ ideas and subsequent improvements are often driven by medical device companies (see Figure 3). In addition, research in non-medical fields may lead to dual use of a device. One example is the MRI machine, which is now used in the diagnosis and monitoring of brain diseases, cancer and trauma (injuries sustained after an accident, for example, a car crash). The technology for it was originally used to study the structure of atoms.An example of the evolution of medical devices is in gastrointestinal endoscopy, a process whereby the clinician can investigate the digestive tract using an endoscope while the patient is usually sedated. First developed in the early nineteenth century, the endoscope now typically consists of a rigid or flexible tube incorporating a light at the end to enable images of the insides of the intestines to be transmitted to the doctor. In the early 2000s, a new process for endoscopy has been developed. The patient swallows a two cm capsule, containing a light source and camera, which passes through the intestines and transmits images as it goes to the clinician. A remote-controlled version is currently being developed (2014). No sedation is required and the patient experience is improved considerably.
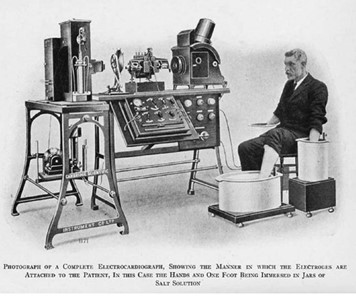
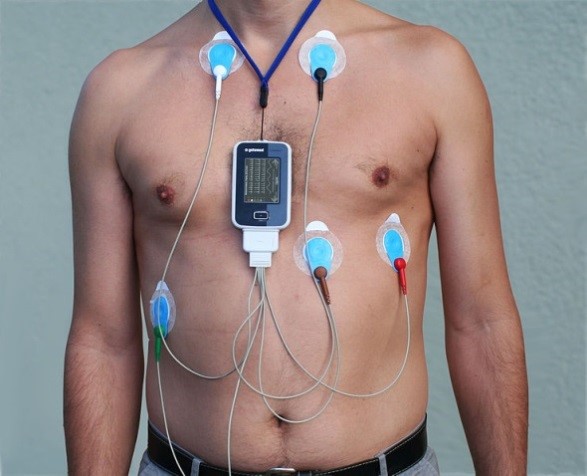
Figure 3. Evolution of the electrocardiograph machine. The left image is an example of an early commercial electrocardiograph (ECG). Contrast this with the modern Holter ECG shown on the right. This is a portable device that can remotely relay real-time ECGs to a medical centre while the patient goes about their everyday life (images courtesy of Wikipedia).
4. Approval Process
5. References
- World Health Organization. Definition of the terms ‘Medical Device’ and ‘In Vitro Diagnostic (IVD) Medical Device’, Global Harmonization Task Force, 2012. Available in Section 2 from: 17158_WHO Global Model Regulatory Framework For Web (Accessed 3 March 2022).
- European Commission . European Council Directive: Medical Devices. 14 June 1993. Available from: EUR-Lex - 31993L0042 - EN - EUR-Lex (europa.eu) (Accessed 23 February 2022).
- World Health Organization. Medical devices: managing the mismatch, 2010. Available from: 9789241564045_eng.pdf (who.int) (Accessed 3 March 2022).
- World Health Organization. Medical device regulations: global overview and guiding principles. Geneva, World Health Organization, 2003. Available from: Medical device regulations : global overview and guiding principles (who.int)