Biologics
| Site: | EUPATI Open Classroom |
| Course: | Types of Medicines and Their Mode of Action and Use |
| Book: | Biologics |
| Printed by: | Guest user |
| Date: | Thursday, 26 June 2025, 12:57 AM |
1. General Features
(This section is organised in the form of a book, please follow the blue arrows to navigate through the book or by following the navigation panel on the right side of the page.)
Biologic medicines, which are also known as biotechnology medicines, biopharmaceutical medicines or biotherapeutic medicinal products, are relatively new, with the first being approved for human use in 1982 (recombinant human insulin).
Today, biologic medicines are used for the diagnosis, treatment and prevention of numerous diseases, including cancer, diabetes mellitus, heart attack, stroke, rheumatoid arthritis and multiple sclerosis. They are usually prescribed by a specialist rather than a primary care physician or family doctor – and are commonly administered in a hospital setting. They are rarely available without a doctor’s prescription.
Biologic proteins are much larger and more complex molecules than traditional chemical medicines; this means they cannot be manufactured as a tablet, so they need to be administered via an injection.
Biologic medicines may come as a powder that needs to be dissolved in a solution before the injection is given or they may already be dissolved in an injectable solution, and may even come in a pre-filled syringe. Most biologic medicines need to be kept cold until they are given to the patient (during both transport and storage).
Some biologic medicines should be given at the same dose to all patients, whereas for others the dose varies according to the patient’s bodyweight. Injections can be administered by a doctor, but in some cases patients or family members may be trained to give the injection. The frequency of dosing depends on the disease and the particular type of biologic medicine administered; it can range from multiple injections per day to just a few injections per year. Similarly, the duration of treatment will also vary and may range from a single course of treatment (possibly comprising administration of multiple doses) to continued treatment for the remainder of a patient’s life.
Biologics are designed to have very specific effects and to interact with specific targets in the patient’s body, mainly on the outside of cells. A more targeted mechanism of action should lead to a greater chance of the medicine having the desired effect against the disease and should result in fewer side effects than traditional medicines. One common side effect of biologics, however, is the risk of immune reactions (immunogenicity), whereby the patient’s immune system recognises the biologic as a ‘foreign’ protein and tries to destroy it. This type of immune reaction may stop the biologic from working entirely or may just cause an irritation at the injection site.
2. Production Process
Biologic medicines are produced using living cells in which many copies of the required proteins are made. Proteins are produced from pieces (known as sequences) of the genetic material deoxyribonucleic acid (DNA), which is found in cells. A sequence of DNA is identified that produces the chosen protein, or parts of the protein, that will be used to diagnose, treat or prevent a particular disease. This protein made from the DNA sequence will form the active component of the biologic medicine. The DNA sequence is inserted into a carrier known as a vector, which then transfers the DNA sequence into a living cell. Each cell that receives the DNA vector will produce a copy of the missing or non-functional protein, and these cells are grown in containers under conditions that allow them to grow in number and produce large quantities of the protein. The proteins are then extracted from the cells, purified and tested for quality. Subsequently, the purified protein is converted into a formulation (e.g. powder or liquid) that can be used as a medicine.
3. Mode of Administration
Most biological medicines cannot be taken as tablets because enzymes in the digestive system break down proteins and therefore, they are usually administered via an injection under the skin (subcutaneous ), into a muscle (intramuscular) or directly into the blood through a vein (intravenous).
4. Example - Insulin
5. Example - Growth factors
Growth factors are proteins made in the body that control the growth and development of cells. Growth factors will typically increase the rate of cell division and may also affect the function of cells and cause them to move to areas of the body where they are needed.Therapeutic growth factors are most commonly used to treat patients with low numbers of certain blood cells, for example low white blood cells (neutropenia), red blood cells (anaemia) and platelets (thrombocytopenia). Growth factors act specifically by binding to matching receptors on the outside of cells. Therefore, general side effects should be limited to immune reactions, such as injection site reactions.
Granulocyte-colony stimulating factor
White blood cells are an important part of the immune system, being involved in fighting infections and other diseases. Granulocyte-colony stimulating factor (G-CSF) is made naturally in the body and maintains the number of white blood cells at normal levels by stimulating their production. Some chemotherapy medicines used to treat patients with cancer cause the levels of white blood cells to drop to dangerously low levels, increasing the chance of infection. Treatment with additional G-CSF helps to prevent this side effect by increasing white blood cell counts.
Chemotherapy is given in cycles, commonly four weeks in duration. G-CSF is given at the same time as chemotherapy and is administered for as long as white blood cell counts are expected to be low. It is available in two different formulations: one that is administered daily and one that is administered just once per chemotherapy cycle.
The benefit of additional G-CSF in patients treated with chemotherapy is that it helps to prevent infections in people who are already seriously ill from their disease. Such infections can be life-threatening and can also lead to the full dose of chemotherapy not being received.
The most common side effect of additional G-CSF is bone or muscle pain, which can be severe in some cases but can usually be controlled with standard painkillers. Other side effects include nausea or vomiting, and rash at the injection site.
6. Example - Antibodies
Antibodies are specialised proteins that are produced by our immune system in response to an attack by germs and other foreign bodies called antigens. Monoclonal antibodies are biologic medicines that are designed to mimic the natural antibodies produced by the body.Monoclonal antibodies are much larger than other protein biologics, such as insulin and growth factors, and the manufacturing process is more complicated. They are produced in the laboratory and designed to bind specifically to other proteins in the body. Some of these proteins may be causing or worsening a disease, and a monoclonal antibody may be administered to block the actions of these proteins. In other scenarios, the monoclonal antibody may bind to a protein on a particular type of cell (such as a cancer cell), causing the cell to die or to be attacked by the body’s own immune system (Figure 1).
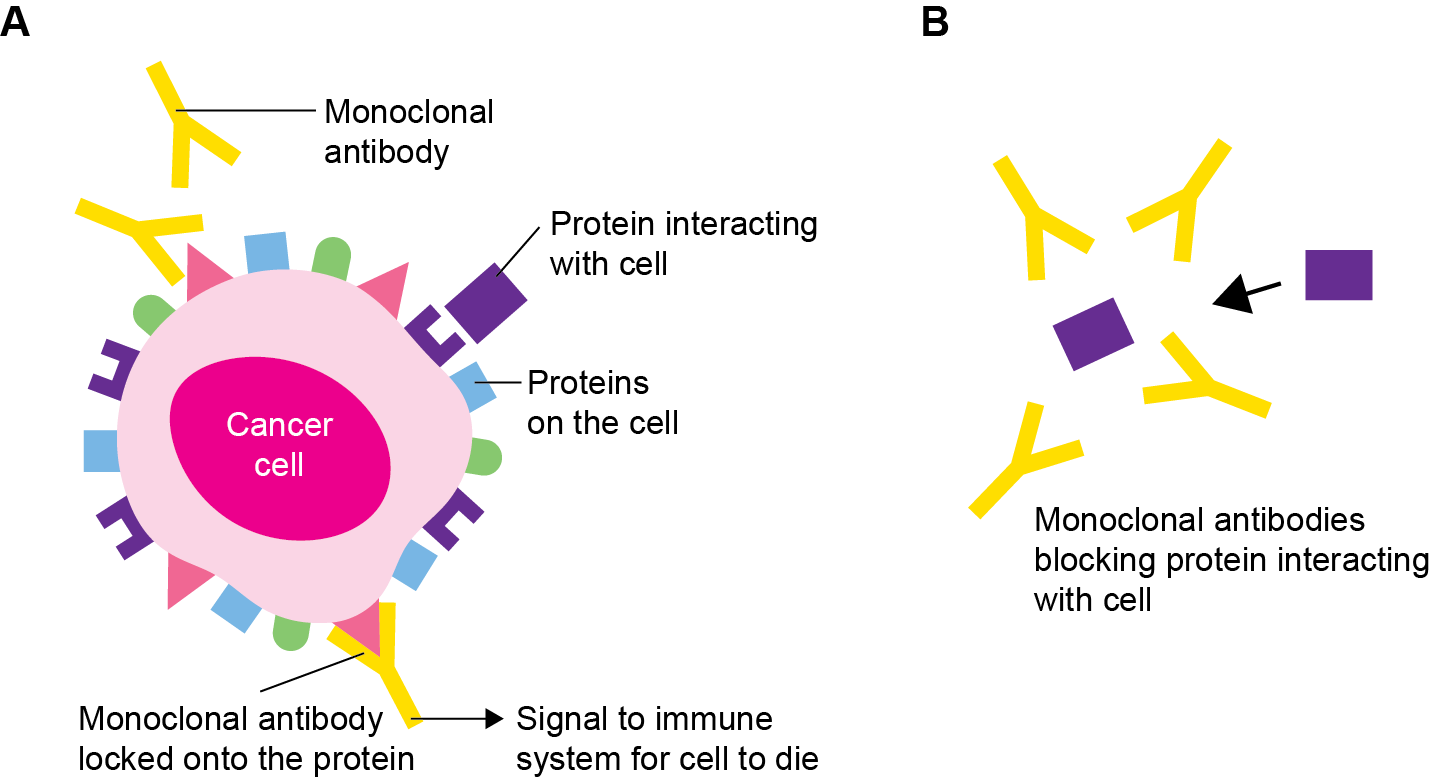
Figure 1. Monoclonal antibodies bind specifically to their target proteins. They can (a) signal for cells to be killed, in the case of cancer cells, or (b) prevent proteins from interacting with cells by binding to them. Adapted from (Link) Monoclonal antibodies | Targeted cancer drugs | Cancer Research UK
Monoclonal antibodies can also be combined with radioactive particles or other toxic medicines, and can deliver these agents to the diseased cells while sparing surrounding healthy cells.
Of the monoclonal antibodies in clinical development, most are designed for use in cancer and immunological disorders. Monoclonal antibodies are delivered by injection into a vein. The number and frequency of injections or infusions will depend on the monoclonal antibody given and the disease. Given that monoclonal antibodies act very specifically, side effects are limited (because there is less interaction with cells or organs that do not carry the target protein). Proteins involved in the disease, however, may also have important functions elsewhere in the body, and so each monoclonal antibody will have its own list of specific side effects. General side effects of monoclonal antibodies are related to allergic reactions to the medicine, and can cause symptoms including fever, rash and headache.
7. Example - Enzymes
Enzymes are a class of protein that catalyse biological
reactions (i.e. they increase the rate at which biological reactions take place). Enzymes catalyse a wide variety of reactions in the body, including those important for digestion (to break down food and to absorb nutrients) and metabolism
(to maintain and build new cells).
In enzymatic reactions, starting molecules (substrates) are converted into the desired product. Enzymes can catalyse specific reactions by interacting with specific substrates – the so-called ‘lock and key’ hypothesis (Figure 2).
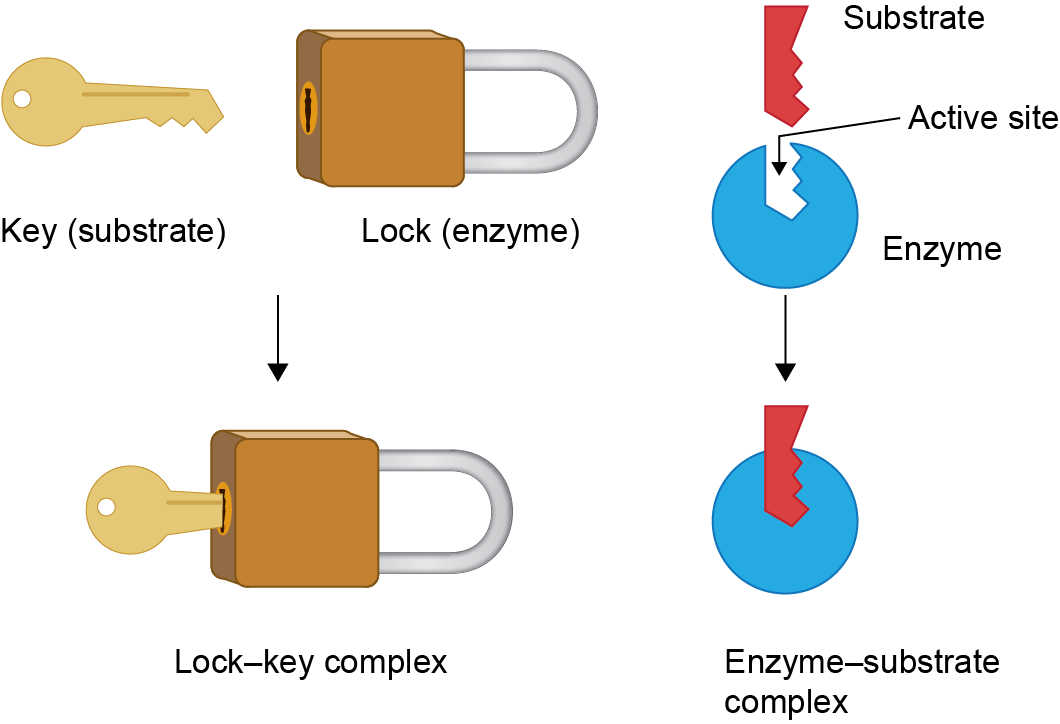
Figure 2. The lock and key hypothesis of enzyme–substrate binding. The shape of the substrate allows it to fit perfectly into the active site of the enzyme, where the enzymatic reaction will take place.
Enzyme dietary supplements are not classical biologic medicines. They are taken as tablets, and instead of being synthesised in the laboratory, they can be extracted directly from animal tissues and purified. Other therapeutic enzymes, however, are developed and manufactured in the same way as other biologic medicines and are administered by injection.
Some diseases (such as Haemophilia, Pompe, Gaucher) are caused by the inability of the body to produce enough of a particular enzyme. Therefore, enzyme therapy is given to restore the amount of enzyme to normal levels.
8. Example - Fusion proteins
By joining together different proteins that have different beneficial qualities, the potency, stability and specificity of fusion proteins can be greatly enhanced compared with naturally occurring proteins. Two types of fusion protein, receptor fusion proteins and peptibodies, have been developed for therapeutic use and other novel variations are being studied.
Receptor fusion proteins join together one or more receptors with part of a naturally occurring antibody. The receptors give the fusion protein its specificity and carry out the desired therapeutic function. For example, the receptors bind to a disease-causing protein and stop it from working and the antibody fragment improves the stability of the molecule (i.e. it increases the amount of time that receptor fusion proteins stay in the body before they are inactivated).
Peptibodies consist of a protein component (or part of a protein, a peptide: ‘pepti-‘) and an antibody-like component (‘-body’). The protein component is the functional part of the peptibody and binds to cells or other proteins to exert its therapeutic effects. As with receptor fusion proteins, the antibody component increases the stability of the molecule as a whole.
An even more recent class of fusion protein joins together two different antibodies. Each of the antibodies attaches to a different type of cell, bringing the two cells into close proximity of one another. In one example, the fusion protein brings together a cell from the immune system with a cancer cell, resulting in the cancer cell being killed by the immune cell.
Similarly to monoclonal antibodies, fusion proteins are likely to be relatively large molecules that require a complex manufacturing and purification process. Fusion proteins are delivered by injection into a vein. The number and frequency of infusions will depend on the fusion protein given and the disease being treated.
Fusion proteins are used to treat a wide variety of diseases, including osteoporosis, cancer and non-cancerous blood disorders. Given their specificity, the number of side effects against cells or organs that are not targeted by the fusion protein should be limited. The targets of fusion proteins involved in disease, however, may also have important functions elsewhere in the body, and so each fusion protein will have its own list of specific side effects. General side effects of fusion proteins are related to allergic reactions to the medicine, and can cause symptoms including fever, rash and headache.
9. References
- European Medicines Agency , 2012. Questions and answers on biosimilar medicines (similar biological medicinal products. Available from: https://www.medicinesforeurope.com/wp-content/uploads/2016/03/WC500020062.pdf (Accessed 3 March 2014).
- US Food and Drug Administration , 2014. What is a biological product? Available from: https://www.fda.gov/files/drugs/published/Biological-Product-Definitions.pdf (Accessed 3 March 2014).
- Mayo Clinic, 2013. Diabetes : definition. Available from: http://www.mayoclinic.org/diseases-conditions/diabetes/basics/definition/con-20033091 (Accessed 3 March 2014).
- Mayo Clinic, 2013. Diabetes: definition. Available from: http://www.mayoclinic.org/diseases-conditions/diabetes/basics/treatment/con-20033091 (Accessed 3 March 2014).
- Patient .co.uk, 2012. Neutropenic patients and neutropenic regimes. Available from: Neutropenic Patients and Neutropenic Regimes (Neutropenia) | Patient (Accessed 3 March 2014).
- Macmillan, 2013. G-CSF (granulocyte-colony stimulating factor). Available from: http://www.macmillan.org.uk/Cancerinformation/Cancertreatment/Treatmenttypes/Supportivetherapies/HaematopoieticGrowthFactors.aspx (Accessed 3 March 2014).
- American Cancer Society, 2013. Monoclonal antibodies. Available from: Monoclonal Antibodies and Their Side Effects | American Cancer Society (Accessed 3 March 2014).
- Mayo Clinic, 2014. Monoclonal antibody drugs for cancer: how they work. Available from: http://www.mayoclinic.org/diseases-conditions/cancer/in-depth/monoclonal-antibody/art-20047808 (Accessed 3 March 2014).